
(5-METHOXYPYRIDIN-2-YL)METHANOL synthesis
- Product Name:(5-METHOXYPYRIDIN-2-YL)METHANOL
- CAS Number:127978-70-5
- Molecular formula:C7H9NO2
- Molecular Weight:139.15
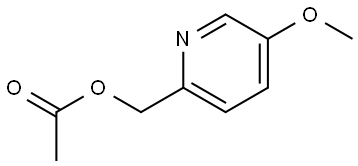
75342-32-4

127978-70-5
Compound 12 (CAS: 75342-32-4, 1.40 g, 7.7 mmol) was used as raw material, which was dissolved in methanol (30 mL) and excess potassium hydroxide was added. The reaction mixture was heated to reflux for 2 hours. After completion of the reaction, the solvent was removed by rotary evaporator under reduced pressure. The residue was extracted by partitioning with dichloromethane (20 mL) and water (20 mL). The organic phase was separated, dried with anhydrous sodium sulfate, filtered and concentrated under reduced pressure to give compound 13 (5-methoxypyridine-2-methanol) as a light yellow oil (1.00 g, 93% yield). The product was characterized by 1H NMR (CDCl3): δ 1.82 (s, 1H), 3.87 (s, 3H), 4.70 (s, 2H), 7.18-7.24 (m, 2H), 8.25 (d, 1H, J = 2.8 Hz); GC/MS analysis showed m/z 140 (M++1, 3%), 139 (M+, 44%), 138 ( 100%), 110 (71%).
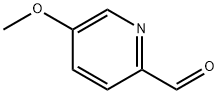
22187-96-8
115 suppliers
$28.00/100mg

127978-70-5
111 suppliers
$29.00/100mg
Yield: 90%
Reaction Conditions:
with sodium tetrahydroborate in methanol at 0; for 0.166667 h;
Steps:
130.130b
To a mixture of 5-methoxypicolinaldehyde (326mgs, 2.36mmol) in MeOH (10 mL) was added NaBH4 (71.9 mg, 1.90 mmol) at O0C. The mixture was stirred at O0C for 10 min after concentration in vacuo, the residue was purified with ISCO MPLC (40-80% EtOAc/hexane) to yield the title compound as a colorless oil (298 mg, 90 %). 1H NMR (CDCl3) 68.28 (d, 1 H), 7.09 - 7.27 (m, 2 H), 4.73 (s, 2 H), 3.89 (s, 3 H). MS (M+H+) = 140.
References:
ASTRAZENECA AB;ASTRAZENECA UK LIMITED WO2009/27746, 2009, A1 Location in patent:Page/Page column 111

75342-32-4
1 suppliers
inquiry

127978-70-5
111 suppliers
$29.00/100mg
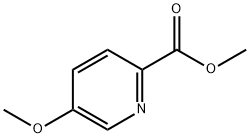
29681-39-8
73 suppliers
$45.00/25mg

127978-70-5
111 suppliers
$29.00/100mg
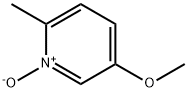
35392-66-6
13 suppliers
inquiry

127978-70-5
111 suppliers
$29.00/100mg
1121-78-4
291 suppliers
$6.00/5g

127978-70-5
111 suppliers
$29.00/100mg