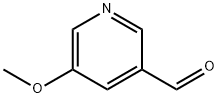
5-METHOXY-PYRIDINE-3-CARBALDEHYDE synthesis
- Product Name:5-METHOXY-PYRIDINE-3-CARBALDEHYDE
- CAS Number:113118-83-5
- Molecular formula:C7H7NO2
- Molecular Weight:137.14
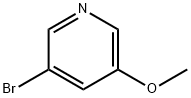
50720-12-2
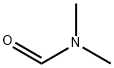
68-12-2

113118-83-5
3-Bromo-5-methoxypyridine (100 mg, 0.53 mmol) was placed in an oven-dried round-bottomed flask equipped with a magnetic stir bar and dissolved in anhydrous tetrahydrofuran (1 mL). Subsequently, isopropylmagnesium chloride (0.3 mL) was added at 0 °C and the resulting mixture was stirred at room temperature for 2 h (the solution turned light brown). Then, anhydrous tetrahydrofuran (0.1 mL) solution of N,N-dimethylformamide (0.1 mL) was slowly added. The initially formed solid gradually dissolved and the color of the solution changed from light brown to light yellow. after 1 h, the reaction mixture was cooled to 0 °C and the reaction was quenched with deionized water (2 mL). The organic layer was separated and the aqueous layer was further extracted with dichloromethane (3 x 3 mL). The organic layers were combined and dried over anhydrous sodium sulfate and subsequently concentrated in vacuum on a rotary evaporator. The crude product was purified by gradient silica gel column chromatography with the eluent being a solvent mixture of hexane and ethyl acetate (ratio from 20:1 to 1:1) to afford the target product 5-methoxy-pyridine-3-carbaldehyde as a colorless slurry (45 mg, 63% yield).1H NMR (500 MHz, CDCl3): δ 10.09 (s, 1H), 8.65 (d, J = 0.9 Hz, 1H), 8.54 (d, J = 3.1 Hz, 1H), 7.60 (dd, J = 5.1, 1.5 Hz, 1H).13C NMR (125 MHz, CDCl3): δ 190.6, 156.2, 145.1, 144.8, 132.0, 116.3, 55.7.

50720-12-2
358 suppliers
$11.00/5g

113118-83-5
146 suppliers
$13.00/250mg
Yield: 155 mg (0.26%)
Reaction Conditions:
with n-butyllithium in tetrahydrofuran;ethyl acetate;N,N-dimethyl-formamide
Steps:
38.b 5-Methoxypyridine-3-carboxaldehyde
b) 5-Methoxypyridine-3-carboxaldehyde. To a stirred solution of 5-bromo-3-methoxypyridine (0.815 g, 4.35 mmol) in THF (15 mL) at -78° C. was added n-BuLi (4.6 mmol). After 1 h, DMF (0.64 g, 8.20 mmol) was added and stirred continuously for 30 min at -78° C. The cold mixture was poured into a stirred aqueous solution of 5% NaHCO3 (25 mL) and extracted with ether (3*15 mL). The extract was evaporated and the crude was purified by chromatography on silica gel with hexane: EtOAc (4:1~2:1) as eluant, yielding 155 mg (0.26%) of the title compound. 1H NMR (CD3OD): 9.88 (s, 1H), 8.44 (s, 1H), 8.32 (s, 1H), 3.70 (s, 3H).
References:
Cytovia, Inc. US2003/65018, 2003, A1
937202-11-4
64 suppliers
$35.00/100mg

113118-83-5
146 suppliers
$13.00/250mg
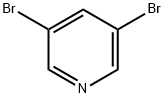
625-92-3
500 suppliers
$6.00/10g

113118-83-5
146 suppliers
$13.00/250mg
113118-74-4
0 suppliers
inquiry

113118-83-5
146 suppliers
$13.00/250mg
20826-01-1
8 suppliers
inquiry

113118-83-5
146 suppliers
$13.00/250mg