
5-bromo-1H-pyrazolo[3,4-c]pyridine synthesis
- Product Name:5-bromo-1H-pyrazolo[3,4-c]pyridine
- CAS Number:929617-35-6
- Molecular formula:C6H4BrN3
- Molecular Weight:198.02

156118-16-0
![5-bromo-1H-pyrazolo[3,4-c]pyridine](/CAS/GIF/929617-35-6.gif)
929617-35-6
General procedure for the synthesis of 5-bromo-1H-pyrazolo[3,4-c]pyridine from 2-bromo-4-methyl-5-aminopyridine: to a solution of 2-bromo-4-methyl-5-aminopyridine (4.00 g, 21.4 mmol) in acetic acid (300 ml) was added sodium nitrite (1.48 g, 21.4 mmol) and the reaction mixture was stirred at room temperature overnight. After completion of the reaction, the mixture was concentrated under vacuum. The residue was diluted with ethyl acetate and washed sequentially with saturated aqueous sodium bicarbonate solution and brine. The organic layer was dried over anhydrous magnesium sulfate and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (eluent: 0-50% hexane solution of ethyl acetate) to afford 5-bromo-1H-pyrazolo[3,4-c]pyridine as a yellow solid (2.48 g, 59% yield).1H NMR (300 MHz, CDCl3): δ 7.86-7.90 (m,1H), 8.09-8.14 (m,1H) 8.83-8.88 (m, 1H). ms ESI/APCI Dual m/z: 198 [M + H].

156118-16-0
152 suppliers
$11.00/250mg
![5-bromo-1H-pyrazolo[3,4-c]pyridine](/CAS/GIF/929617-35-6.gif)
929617-35-6
165 suppliers
$9.00/100mg
Yield:929617-35-6 59%
Reaction Conditions:
with acetic acid;sodium nitrite at 20;
Steps:
Synthesis of 5-bromo-1H-pyrazolo[3,4-c]pyridine (28)
To a solutionof 27(4.00 g, 21.4 mmol) in AcOH (300 ml) was added NaNO2 (1.48 g, 21.4mmol) and stirred overnight at roomtemperature. The reaction mixture was concentrated in vacuo. The residue was diluted with EtOAc and washed with saturatedNaHCO3 aqueous solution and brine. Theorganic layer was dried over anhydrous MgSO4 and reduced underpressure. The residue was purified bysilica gel column chromatography (0-50% EtOAc in hexanes) to afford 28 as a yellow solid (2.48 g, 59%yield).1H NMR (300 MHz, CDCl3): δ ppm7.86 - 7.90 (m, 1 H), 8.09 - 8.14 (m, 1 H), 8.83 - 8.88 (m, 1 H).MS ESI/APCI Dual m/z: 198 [M+H] .
References:
Matsuda, Daisuke;Kobashi, Yohei;Mikami, Ayako;Kawamura, Madoka;Shiozawa, Fumiyasu;Kawabe, Kenichi;Hamada, Makoto;Oda, Koji;Nishimoto, Shinichi;Kimura, Kayo;Miyoshi, Masako;Takayama, Noriko;Kakinuma, Hiroyuki;Ohtake, Norikazu [Bioorganic and Medicinal Chemistry Letters,2016,vol. 26,# 15,p. 3441 - 3446] Location in patent:supporting information

156118-16-0
152 suppliers
$11.00/250mg

543-87-3
69 suppliers
$20.00/1g
![5-bromo-1H-pyrazolo[3,4-c]pyridine](/CAS/GIF/929617-35-6.gif)
929617-35-6
165 suppliers
$9.00/100mg
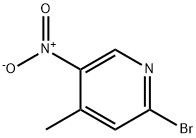
23056-47-5
270 suppliers
$10.00/1g
![5-bromo-1H-pyrazolo[3,4-c]pyridine](/CAS/GIF/929617-35-6.gif)
929617-35-6
165 suppliers
$9.00/100mg