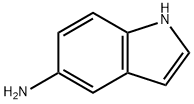
5-Aminoindole synthesis
- Product Name:5-Aminoindole
- CAS Number:5192-03-0
- Molecular formula:C8H8N2
- Molecular Weight:132.16

6146-52-7

5192-03-0
In Example 1, 5-nitroindole was substituted for m-nitroacetophenone as a raw material, maintaining a molar ratio of 1:1. The reaction was carried out at 80 °C for 24 h under hydrogen pressure of 4.0 MPa. Other conditions were consistent with Example 1, and 5-aminoindole was finally obtained in 98% yield. This was done as follows: 16.44 mg (0.04 mmol) of silver 4,4'-dimethoxy-2,2'-bipyridine, 11.22 mg (0.1 mmol) of potassium tert-butanolate and 1 ml of 1,4-dioxane solution were added to an autoclave. After thorough stirring, 165.15 mg (1 mmol) of m-nitroacetophenone was added and the mixture was stirred and reacted at 80 °C for 8 hours. Upon completion of the reaction, the reaction solution was extracted with deionized water and dichloromethane and the organic phase was collected. The organic phase was dried over anhydrous Na2SO4 and then subjected to filtration, rotary evaporation and chromatographic separation to afford 3-acetanilide as a yellow solid in 96% yield.

6146-52-7
468 suppliers
$13.00/1g

5192-03-0
328 suppliers
$14.14/250mgs:
Yield:5192-03-0 99%
Reaction Conditions:
with C20H32Cl4Cu2N4O4 in water at 60; for 2 h;Inert atmosphere;Schlenk technique;
Steps:
2.4. General procedure for the reduction of nitro compounds withcopper complex catalyst
General procedure: Copper complex (0.001 mmol, 1mol%), appropriate nitro compounds (0.1 mmol, 1.0 equiv) and NaBH4 (10.0 mmol, 10 equiv)in water (2.0 mL) were charged into a 15 mL pressure tube under air atmosphere. Subsequently, the resulting mixture was stirred atroom temperature in closed vessel. After completion of the reaction(monitored by TLC), the crude reaction mixturewas extraction with ether (3 2 mL), and analyzed by GC-MS chromatography using n-dodecaneas an internal standard.
References:
Du, Jun;Gao, Li-Li;Jia, Wei-Guo;Li, Mei;Zhi, Xue-Ting [Journal of Molecular Structure,2020,vol. 1217]
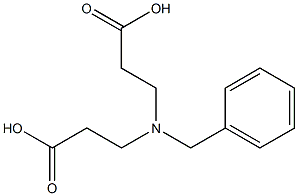
6405-28-3
30 suppliers
$90.00/500mg

6146-52-7
468 suppliers
$13.00/1g

5192-03-0
328 suppliers
$14.14/250mgs:

10075-50-0
690 suppliers
$5.00/10g

5192-03-0
328 suppliers
$14.14/250mgs:

32692-19-6
193 suppliers
$10.00/250mg

5192-03-0
328 suppliers
$14.14/250mgs:

166104-20-7
132 suppliers
$13.00/100mg

5192-03-0
328 suppliers
$14.14/250mgs: