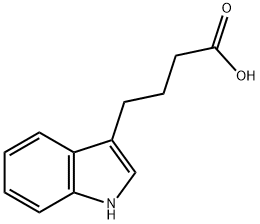
Indole-3-butyric acid synthesis
- Product Name:Indole-3-butyric acid
- CAS Number:133-32-4
- Molecular formula:C12H13NO2
- Molecular Weight:203.24
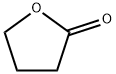
96-48-0
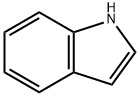
120-72-9

133-32-4
Indole (11.7 g), γ-butyrolactone (11.2 g), tetrahydronaphthalene (58.5 g), polyethylene glycol (loading 17% of hydrazine) and Na-NaOH/γ-Al2O3 (loading 5% of hydrazine) were added sequentially to the reactor. A stirrer was activated and the reaction was stirred for 30 min at room temperature to mix the reactants homogeneously. Subsequently, the reaction system was heated to 210 °C for reflux reaction. After 5 hours of reaction, the heating was stopped and the solid catalyst was removed by filtration. The filtrate was cooled, the organic layer was separated and acidified with hydrochloric acid to obtain an off-white solid. After filtration, washing with water and drying, 3-indolebutyric acid (18.2 g) was obtained. The above reaction conditions were repeated using the recovered catalyst to catalyze the reaction of indole and γ-butyrolactone to give 3-indolebutyric acid (17.7 g).
Yield:133-32-4 90%
Reaction Conditions:
with Na-NaOH/γ-Al2O3;polyethylene glycol in tetralin at 210; for 5 h;
Steps:
2 Embodiment 2
Indole, γ-butyrolactone, tetrahydronaphthalene, polyethylene glycol and Na-NaOH/y-Al203 were added to the reactor, wherein the loading amounts of ruthenium, γ-butyrolactone and tetrahydronaphthalene were respectively 11.7 g, 11.2 g, and 58.5 g, the loading amount of polyethylene glycol was 17% of hydrazine, and the loading amount of Na-Na0H/y-Al203 was 5% of hydrazine. Start the stirring function of the heating stirrer and stir for 30 minutes at room temperature to mix the five uniformly. Then, start the heating function of the heating stirrer and heat it to 210 ° C to carry out the heating and reflux reaction. After the reaction for 5 hours, the heating is stopped, and the filter residue is a solid catalyst. The mixture was recovered, the filtrate was cooled, and the organic layer was separated and acidified with hydrochloric acid to give an off-white solid, which was filtered, washed with water, and dried to give 18.2 g of the final product 3-indole acid in a yield of 90%.
Utilizing the above-mentioned recovery obtained catalyst, once again for the catalytic indole and γ - butyrolactone in the reaction, the reaction conditions are not changed, to obtain the final product 3 - indole butyric acid 17.7 g.
References:
CN108440371,2018,A Location in patent:Paragraph 0027-0028; 0029; 0031-0032; 0035-0036; 0039-0040
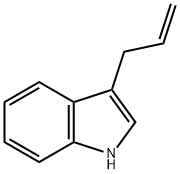
16886-09-2
47 suppliers
$72.00/100mg
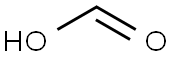
64-18-6
1127 suppliers
$22.12/250ML

133-32-4
681 suppliers
$10.00/5g

31529-29-0
5 suppliers
$28.60/250mg

133-32-4
681 suppliers
$10.00/5g
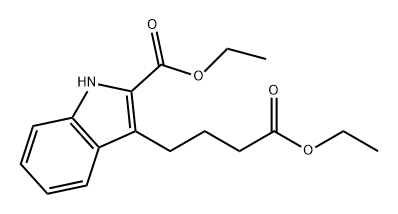
63183-76-6
0 suppliers
inquiry

133-32-4
681 suppliers
$10.00/5g
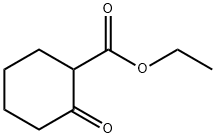
1655-07-8
308 suppliers
$6.00/5g

133-32-4
681 suppliers
$10.00/5g