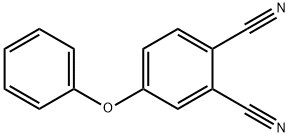
4-PHENOXYPHTHALONITRILE synthesis
- Product Name:4-PHENOXYPHTHALONITRILE
- CAS Number:38791-62-7
- Molecular formula:C14H8N2O
- Molecular Weight:220.23
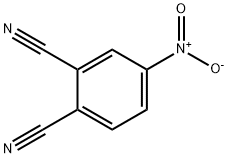
31643-49-9
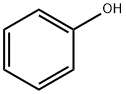
108-95-2

38791-62-7
General procedure for the synthesis of 4-phenoxyphthalonitrile from 4-nitrophthalonitrile and phenol: To a solution of tetrahydrofuran (THF, 15 mL) containing sodium hydride (NaH, 0.15 g) and phenol (0.353 g, 0.0375 mol), slowly add 4-nitrophthalonitrile (0.50 g, 0.0028 mol) to the THF (15 mL ) solution. The reaction mixture was heated to reflux for 8 hours. Upon completion of the reaction, 1,2-diisocyano-4-phenoxybenzene (8) was obtained by filtration and the solid was washed with ethyl acetate. The filtrate and washings were combined and the solvent was removed by evaporation under reduced pressure. The white precipitate obtained was purified by fast column chromatography using ethyl acetate: hexane (1:4) as eluent. The yield was 0.737 g in 86.4% with an Rf value of 0.36 (ethyl acetate: hexane, 1:4).1H NMR (300 MHz, CDCl3) δ 0.90 (s, 2H), 3.46 (m, 5H, CH), 7.07 (m, 3H, ArH).13C NMR (300 MHz, CDCl3) δ 71.01, 117.45, 119.69, 119.92, 126.95, 127.34, 134.43, 135.20.

31643-49-9
462 suppliers
$6.00/5g

108-95-2
770 suppliers
$14.00/25g

38791-62-7
71 suppliers
$22.00/250mg
Yield: 86.4%
Reaction Conditions:
with sodium hydride in tetrahydrofuran for 8 h;Inert atmosphere;Reflux;
Steps:
Synthesis of 2,3,9,10,16,17,23,24-octa substituted phthalocyanine (2a)
To NaH (0.15 g) and phenol, (0.353 g,0.0375 mol) in THF (15 mL) was added and 4-nitrophthalonitrile(0.50 g, 0.0028 mol), in THF (15 mL) and refluxed for 8 h. The resulting 1,2-diisocyano-4-phenoxybenzene (8) was filtrated and the solid washed with ethyl acetate and both the filtrate and the washing were mixed and evaporated under reduced pressure (Scheme-II). The resulting white precipitate was purified by flash column chromatography using solvent system, ethyl acetate:hexane (1:4). Yield = 0.737 g, % Yield =86.4, Rf = 0.36 (ethyl acetate:hexane, 1:4). 1H NMR (300 MHz, CDCl3) δ 0.90 (s, 2H), 3.46 (m, 5H,CH) 7.07 (m, 3H, ArH), 13C NMR (300 MHz, CDCl3) δ 71.01,117.45, 119.69, 119.92, 126.95, 127.34, 134.43, 135.20.
References:
Isabirye, David A.;Seheri, Naledi H.;Aiyelabola, Temitayo O. [Asian Journal of Chemistry,2017,vol. 29,# 3,p. 489 - 495]

31643-49-9
462 suppliers
$6.00/5g
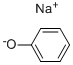
139-02-6
139 suppliers
$34.00/5g

38791-62-7
71 suppliers
$22.00/250mg