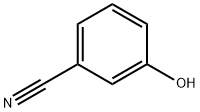
3-Cyanophenol synthesis
- Product Name:3-Cyanophenol
- CAS Number:873-62-1
- Molecular formula:C7H5NO
- Molecular Weight:119.12
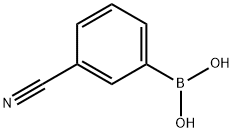
150255-96-2

873-62-1
The general procedure for the synthesis of 3-hydroxybenzonitrile from 3-cyanophenylboronic acid was as follows: arylboronic acid (1 mmol), 30% H2O2 aqueous solution (0.2 mL), ZnO nanocatalyst (5 mol%, sample ZnO-1), and 2 mL of water were mixed with stirring in a 50 mL round bottom flask at room temperature and under aerobic conditions. The reaction process was monitored by thin layer chromatography (TLC). Upon completion of the reaction, the reaction mixture was diluted with 20 mL of water and extracted with diethyl ether (3 x 20 mL). The organic layers were combined, washed with brine and dried over Na2SO4. Subsequently, the solvent was removed under reduced pressure in a rotary evaporator. The crude product was purified by silica gel (100-200 mesh) column chromatography (eluent: hexane/ethyl acetate, 9:1) to afford the target product 3-hydroxybenzonitrile. The structure of the product was confirmed by 1H NMR and 13C NMR.
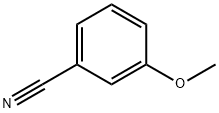
1527-89-5
292 suppliers
$6.00/5g

873-62-1
265 suppliers
$6.00/1g
Yield:873-62-1 90%
Reaction Conditions:
with water;hydrogen bromide;Aliquat 336 at 105; for 6.5 h;Catalytic behavior;
Steps:
General experimental procedure for deprotection of aryl methyl ether
General procedure: To a stirred solution of aryl methyl ether (20 mmol) and 47% aqueous HBr (4.5 mmol equiv. of substrate) added Aliquat-336 (10 wt. % of substrate) in a single lot. The resulting reaction mixture was heated at 105±5 °C, and the progress of reaction was monitored by TLC. After completion of reaction, the reaction mixture was cooled to room temperature and quenched by adding water (25 ml). The resulting reaction mass was extracted with 3x30 ml ethyl acetate. Ethyl acetate layer washed twice with 20 ml of water, dried over anhydrous Na2SO4 and evaporated under reduced pressure. The crude product was purified by silica gel column chromatography using ethyl acetate/hexane system.
References:
Waghmode, Suresh B.;Mahale, Ganesh;Patil, Viraj P.;Renalson, Kartik;Singh, Dharmendra [Synthetic Communications,2013,vol. 43,# 24,p. 3272 - 3280] Location in patent:supporting information

150255-96-2
365 suppliers
$8.00/1g

873-62-1
265 suppliers
$6.00/1g

100-83-4
654 suppliers
$5.00/10g

873-62-1
265 suppliers
$6.00/1g

22241-18-5
11 suppliers
$110.00/50mg

873-62-1
265 suppliers
$6.00/1g
6952-59-6
366 suppliers
$9.00/10g

873-62-1
265 suppliers
$6.00/1g