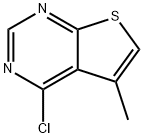
4-CHLORO-5-METHYLTHIENO[2,3-D]PYRIMIDINE synthesis
- Product Name:4-CHLORO-5-METHYLTHIENO[2,3-D]PYRIMIDINE
- CAS Number:43088-67-1
- Molecular formula:C7H5ClN2S
- Molecular Weight:184.65

43088-64-8
![4-CHLORO-5-METHYLTHIENO[2,3-D]PYRIMIDINE](/CAS/GIF/43088-67-1.gif)
43088-67-1
Step 2. Preparation of 4-chloro-5-methylthieno[2,3-d]pyrimidine: 5-methylthieno[2,3-d]pyrimidin-4(3H)-one (1.4 g, 8.42 mmol) prepared in Step 1 was dissolved in phosphorochloridic acid (5 mL). Subsequently, the reaction mixture was stirred at 110°C for 1 hour. Upon completion of the reaction, the reaction mixture was slowly poured into a mixture of ice water and chloroform and washed sequentially with water and saturated aqueous sodium bicarbonate. The organic layers were combined and concentrated under reduced pressure. The residue was purified by recrystallization (hexane/ethyl acetate mixed solvent) to give 590 mg (yield: 39%) of the target product 4-chloro-5-methylthieno[2,3-d]pyrimidine as a yellow solid.1H NMR (400 MHz, CDCl3): δ 8.81 (s, 1H), 7.24 (s, 1H), 2.70 (s, 3H).

43088-64-8
37 suppliers
$90.00/100mg
![4-CHLORO-5-METHYLTHIENO[2,3-D]PYRIMIDINE](/CAS/GIF/43088-67-1.gif)
43088-67-1
96 suppliers
$16.00/250mg
Yield:43088-67-1 39%
Reaction Conditions:
with trichlorophosphate at 110; for 1 h;
Steps:
9.2
Step 2. Preparation of 4-chloro-5-methyl-thienor2.3-dlpyrimidine; The compound, 5-methyl-thieno[2,3-d]pyrimidin-4-ol, (1.4g, 8.42mmol) prepared in the step 1 was dissolved in phosphorusoxy chloride (5ml). Thereafter, the reaction mixture was stirred at 11O0C for 1 hour. After reaction, the reaction mixture was poured into ice water and chloroform, and washed with water and saturated sodium hydrogen carbonate aqueous solution. Combined organic layer was concentrated under reduced pressure. The residue was purified by recrystallization (n-hexane and ethyl acetate) to give 590mg (yield: 39%, yellow solid) of the target compound.[514] 1U NMR(400D, CDCI): δ 8.81 (s, IH), 7.24 (s, IH), 2.70 (s, 3H).
References:
WO2007/102679,2007,A1 Location in patent:Page/Page column 49

43088-64-8
37 suppliers
$90.00/100mg
![4-CHLORO-5-METHYLTHIENO[2,3-D]PYRIMIDINE](/CAS/GIF/43088-67-1.gif)
43088-67-1
96 suppliers
$16.00/250mg

43088-42-2
174 suppliers
$5.00/250mg
![4-CHLORO-5-METHYLTHIENO[2,3-D]PYRIMIDINE](/CAS/GIF/43088-67-1.gif)
43088-67-1
96 suppliers
$16.00/250mg