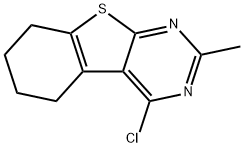
4-CHLORO-2-METHYL-5,6,7,8-TETRAHYDRO[1]BENZOTHIENO[2,3-D]PYRIMIDINE synthesis
- Product Name:4-CHLORO-2-METHYL-5,6,7,8-TETRAHYDRO[1]BENZOTHIENO[2,3-D]PYRIMIDINE
- CAS Number:81765-97-1
- Molecular formula:C11H11ClN2S
- Molecular Weight:238.74
![2-methyl-5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidin-4(3H)-one](/CAS/20180528/GIF/19819-15-9.gif)
19819-15-9
![4-CHLORO-2-METHYL-5,6,7,8-TETRAHYDRO[1]BENZOTHIENO[2,3-D]PYRIMIDINE](/CAS/GIF/81765-97-1.gif)
81765-97-1
GENERAL METHOD: Different thieno[2,3-d]pyrimidin-4-one derivatives (6a-d or f-i) (1 eq.) were mixed with phosphorochloridic acid chloride (2.9 mL/mmol eq.) and heated to reflux for 8 hours. Upon completion of the reaction, the reaction mixture was cooled in an ice bath and neutralized by slow addition of saturated aqueous sodium bicarbonate solution. Subsequently, the reaction mixture was extracted with ethyl acetate (15 mL/millimolar equivalent), the organic layer was separated, dried with anhydrous magnesium sulfate and concentrated under reduced pressure. The crude product was purified by fast column chromatography with the eluent being n-hexane-ethyl acetate (initial ratio 100:0 v/v), which was gradually adjusted to n-hexane-ethyl acetate (85:15 v/v), unless otherwise stated.
![2-methyl-5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidin-4(3H)-one](/CAS/20180528/GIF/19819-15-9.gif)
19819-15-9
10 suppliers
inquiry
![4-CHLORO-2-METHYL-5,6,7,8-TETRAHYDRO[1]BENZOTHIENO[2,3-D]PYRIMIDINE](/CAS/GIF/81765-97-1.gif)
81765-97-1
41 suppliers
$44.00/250mg
Yield:81765-97-1 97%
Reaction Conditions:
with trichlorophosphate at 110; for 3 h;
Steps:
3 Intermediate 4
Intermediate 3 (0.82 mmol) and 5 mL of POCl3 were added into a 100 mL evaporating flask. The reaction solution was refluxed under stirring at 110° C. for 3 h, then cooled to room temperature and evaporated to dryness by rotary evaporation under reduced pressure. The residue was dissolved in an appropriate amount of ethyl acetate and adjusted to be alkaline by adding a saturated aqueous NaHCO3 solution. Then the mixture was extracted with ethyl acetate, washed with a saturated NaCl aqueous solution, and the organic layer was evaporated to dryness by rotary evaporation to obtain an Intermediate 4 (white solid, 97% yield), which was directly put into next reaction without purification.
References:
US2022/227782,2022,A1 Location in patent:Paragraph 0059
![2-methyl-5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidin-4(3H)-one](/CAS/20180528/GIF/19819-15-9.gif)
19819-15-9
10 suppliers
inquiry
![4-CHLORO-2-METHYL-5,6,7,8-TETRAHYDRO[1]BENZOTHIENO[2,3-D]PYRIMIDINE](/CAS/GIF/81765-97-1.gif)
81765-97-1
41 suppliers
$44.00/250mg
![ETHYL 2-AMINO-4,5,6,7-TETRAHYDROBENZO[B]THIOPHENE-3-CARBOXYLATE](/CAS/GIF/4506-71-2.gif)
4506-71-2
203 suppliers
$6.00/1g
![4-CHLORO-2-METHYL-5,6,7,8-TETRAHYDRO[1]BENZOTHIENO[2,3-D]PYRIMIDINE](/CAS/GIF/81765-97-1.gif)
81765-97-1
41 suppliers
$44.00/250mg
108-94-1
579 suppliers
$12.00/50g
![4-CHLORO-2-METHYL-5,6,7,8-TETRAHYDRO[1]BENZOTHIENO[2,3-D]PYRIMIDINE](/CAS/GIF/81765-97-1.gif)
81765-97-1
41 suppliers
$44.00/250mg