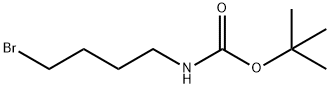
tert-butyl (4-bromobutyl)carbamate synthesis
- Product Name:tert-butyl (4-bromobutyl)carbamate
- CAS Number:164365-88-2
- Molecular formula:C9H18BrNO2
- Molecular Weight:252.15
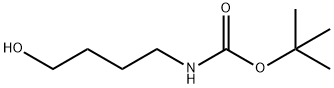
75178-87-9

164365-88-2
General procedure for the synthesis of tert-butyl N-(4-bromobutyl) carbamate from 4-(N-tert-butoxycarbonylamino)-1-butanol: 4-(N-tert-butoxycarbonylamino)-1-butanol (1.06 g, 5.788 mmol) was dissolved in anhydrous THF (54 mL), followed by the addition of triphenylphosphine (2.86 g, 10.92 mmol, 1.9 equiv) . Carbon tetrabromide (3.62 g, 10.92 mmol, 1.9 eq.) was added slowly with stirring. The reaction mixture was stirred at room temperature for 3 h. After stirring, the reaction mixture was filtered through a diatomaceous earth pad to remove by-products and the filter cake was washed with ether. The filtrate was concentrated under reduced pressure to remove the solvent. The crude product was purified by silica gel fast column chromatography (eluent: hexane/ethyl acetate, 3:1) to afford tert-butyl N-(4-bromobutyl)carbamate (1.73 g, quantitative yield) as a white solid.
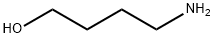
13325-10-5
316 suppliers
$10.00/1g

24424-99-5
865 suppliers
$13.50/25G

164365-88-2
134 suppliers
$31.00/100mg
Yield:164365-88-2 80%
Reaction Conditions:
Stage #1:4-Aminobutanol;di-tert-butyl dicarbonate with triethylamine in acetonitrile at 0; for 6 h;
Stage #2: with carbon tetrabromide;triphenylphosphine in tetrahydrofuran for 3 h;
Steps:
Synthesis of tert-butyl (4-bromobutyl)carbamate (10)
To a solution of 4-amino-1-butanol (1.50 g, 16.8 mmol) in MeCN (150 mL) were added TEA (4.68 mL) and Boc2O (4.03 g, 18.5 mL) at 0 °C. The mixture was stirred for 6 h and quenched with water. The mixture was poured onto water and extracted with EtOAc three times. The combined organic layers were dried over Na2SO4 and concentrated under reduced pressure. The crude product 6 (2.68 g) was used without further purification. The crude product 6 was dissolve in THF (130 mL) followed by addition of Ph3P (7.06 g, 26.9 mmol). Then CBr4 (8.92 g, 26.9 mmol) was slowly added to the mixture. After 3 h, the solution was filtered through Celite? pad and washed with Et20. The solvents were removed under reduced pressure. The residue was purified by flash chromatography on silica (hexane:EtOAc = 3:1) to yield a colourless oil (4.38 g, 80percent).‘H NMR (500 MHz, CDC13) 4.54 (br s, 1H), 3.43 (t, J= 6.7 Hz, 2H), 3.17 (q, J= 6.7 Hz, 2H), 1.90 (dt, J= 14.8, 6.8 Hz, 2H), 1.65 (m, 2H), 1.45 (s, 9H).
References:
UNIVERSITY OF ADELAIDE;ABELL, Andrew;POLYAK, Steven;TIEU, William;BOOKER, Grant;JUN, Kwang Lee;RODRIGEZ, Beatriz Blanco WO2020/87122, 2020, A1 Location in patent:Page/Page column 26; 27

75178-87-9
127 suppliers
$6.00/100mg

164365-88-2
134 suppliers
$31.00/100mg
4509-90-4
235 suppliers
$15.00/1g
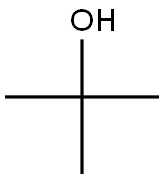
75-65-0
785 suppliers
$10.00/10ml

164365-88-2
134 suppliers
$31.00/100mg

24424-99-5
865 suppliers
$13.50/25G
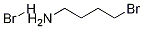
24566-81-2
102 suppliers
$9.00/250mg

164365-88-2
134 suppliers
$31.00/100mg

33977-38-7
10 suppliers
inquiry

24424-99-5
865 suppliers
$13.50/25G

164365-88-2
134 suppliers
$31.00/100mg