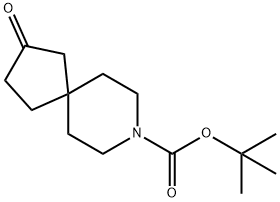
tert-butyl 2-oxo-8-azaspiro[4.5]decane-8-carboxylate synthesis
- Product Name:tert-butyl 2-oxo-8-azaspiro[4.5]decane-8-carboxylate
- CAS Number:1250994-14-9
- Molecular formula:C14H23NO3
- Molecular Weight:253.34
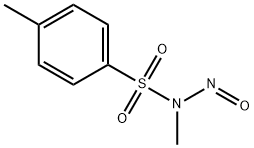
80-11-5
![tert-butyl 2-oxo-7-azaspiro[3.5]nonane-7-carboxylate](/CAS/GIF/203661-69-2.gif)
203661-69-2
![tert-butyl 2-oxo-8-azaspiro[4.5]decane-8-carboxylate](/CAS/GIF/1250994-14-9.gif)
1250994-14-9
A 10% to 40% aqueous solution of potassium hydroxide (20 mL) was added to a solvent mixture of tetrahydrofuran and methanol containing tert-butyl-2-oxo-7-azaspiro[3.5]nonane-7-carboxylic acid (8.2 g). The solvent ratio was 10:1. In another vessel, N-methyl-N-nitroso-p-toluenesulfonamide (19.6 g) was dissolved in 300 mL of water and added slowly and dropwise at 0 °C to the above reaction solution. The reaction mixture was stirred at room temperature for 24 hours. Upon completion of the reaction, the system was cooled to room temperature and the reaction was quenched by the addition of acetic acid. Subsequently, the reaction mixture was rotary evaporated to dryness and the residue was dissolved in ethyl acetate (100 mL) and washed sequentially with water (100 mL) and saturated brine (100 mL). The organic layer was dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. Purification by column chromatography afforded ethyl tert-butyl-2-oxo-8-azaspiro[4.5]decane-8-carboxylate (6.6 g, yield: 72%).

80-11-5
201 suppliers
$160.00/1g
![tert-butyl 2-oxo-7-azaspiro[3.5]nonane-7-carboxylate](/CAS/GIF/203661-69-2.gif)
203661-69-2
173 suppliers
$8.00/250mg
![tert-butyl 2-oxo-8-azaspiro[4.5]decane-8-carboxylate](/CAS/GIF/1250994-14-9.gif)
1250994-14-9
55 suppliers
$257.00/100mg
Yield: 72%
Reaction Conditions:
with potassium hydroxide in tetrahydrofuran;methanol;water at 0 - 20; for 24 h;Temperature;Solvent;
Steps:
3
A solution of 10% to 40% aqueous potassium hydroxide (20 mL) was added to a solution of tert-butyl-2-carbonyl-7-spiro [3.5] nonane-7-carboxylic acid (8.2 g) in tetrahydrofuran and methanol Ratio: 10/1 to 1/10) in a mixed solvent,N-methyl-N-nitro-p-toluenesulfonamide (19.6 g) was dissolved in 300 ml of water,And the mixture was added dropwise to the above reaction solution at 0 ° C, followed by reaction at room temperature for 24 hours. Raw material reaction is complete, the system cooled to room temperature, add acetic acid quenching spin dry,Then dissolved in ethyl acetate (100 mL)Washed with water (100 mL) and saturated brine (100 mL), respectively.Then dried over anhydrous sodium sulfate, filtered, dried under reduced pressure,Tert-butyl-2-carbonyl-8-spiro [4.5] decane-8-carboxylic acid (6.6 g, yield: 72%) was obtained by column chromatography.
References:
CHANGZHOU HEQUAN PHARMACEUTICAL CO LTD;Shanghai Syn-The-All medical research and development Co . ltd;Qian, guolei;Mao, yanjun;Chen, Linlin;Zhou, jiang;Li, Hong;Tang, xiaowu;Yang, caimin;Zhang, TongXin;Sun, Wei;Yu, Leng Bo;He, zhenmin;Ma, RuJian;Chen, minzhang;Fu, XiaoYong;Wang, WenGui CN105272914, 2016, A Location in patent:Paragraph 0010; 0014
684-93-5
172 suppliers
$24.00/250mg
![tert-butyl 2-oxo-7-azaspiro[3.5]nonane-7-carboxylate](/CAS/GIF/203661-69-2.gif)
203661-69-2
173 suppliers
$8.00/250mg
![tert-butyl 2-oxo-8-azaspiro[4.5]decane-8-carboxylate](/CAS/GIF/1250994-14-9.gif)
1250994-14-9
55 suppliers
$257.00/100mg

137076-22-3
353 suppliers
$5.00/1g
![tert-butyl 2-oxo-8-azaspiro[4.5]decane-8-carboxylate](/CAS/GIF/1250994-14-9.gif)
1250994-14-9
55 suppliers
$257.00/100mg