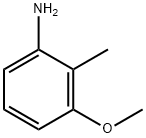
3-Methoxy-2-methylaniline synthesis
- Product Name:3-Methoxy-2-methylaniline
- CAS Number:19500-02-8
- Molecular formula:C8H11NO
- Molecular Weight:137.18
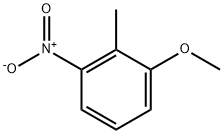
4837-88-1

19500-02-8
The general procedure for the synthesis of 3-methoxy-2-methylaniline from 2-methyl-3-nitroanisole is as follows (reference Example 42): 1. 9.98 g of a suspension of 5% palladium-carbon (wet) in 100 mL of ethanol was added to a solution of 30.7 g (184 mmol) of 2-methyl-3-nitroanisole in 300 mL of ethanol. 2. The reaction mixture was stirred at room temperature and under hydrogen atmosphere for 3 hours. 3. Upon completion of the reaction, the reaction solution was filtered through diatomaceous earth. 4. The filtrate was concentrated under vacuum to give 25.5 g of 3-methoxy-2-methylaniline as a light purple oil (Yield: quantitative). Rf value: 0.38 (hexane: ethyl acetate = 2:1, v/v). Mass spectrum (EI, m/z): 137 (M+). 1H-NMR spectrum (CDCl3, δ ppm): 2.04-2.05 (m, 3H), 3.60 (brs, 2H), 3.80 (s, 3H), 6.33-6.37 (m, 2H), 6.94-7.01 (m, 1H).
77-78-1
326 suppliers
$19.69/1ml

19500-02-8
213 suppliers
$9.00/1g
Yield: 89%
Reaction Conditions:
Stage #1:3-amino-6-iodo-2-methyl-2-cyclohexen-1-one with potassium tert-butylate in tetrahydrofuran at -15 - 5; for 1 h;
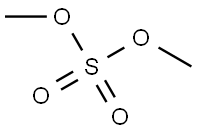
Stage #2:dimethyl sulfate in tetrahydrofuran at 5; for 2 h;
Steps:
2.24 g (20 mmol, 2 eq) of potassium tert-butoxide is dissolved in 60 ml of anhydrous THF and the resulting solution is cooled to -15 0C with stirring. A solution of 2.51 g (10 mmol, 1 eq) of 3-amino-6-iodo-2-methyl-2-cyclohexen-l-one in 25 ml anhydrous THF is added to the potassium tert-butoxide solution and the temperature is kept below
References:
BOEHRINGER INGELHEIM INTERNATIONAL GMBH;BOEHRINGER INGELHEIM PHARMA GMBH & CO. KG WO2007/130704, 2007, A1 Location in patent:Page/Page column 6-7

4837-88-1
237 suppliers
$12.72/1gm:

19500-02-8
213 suppliers
$9.00/1g
5460-31-1
298 suppliers
$6.00/1g

19500-02-8
213 suppliers
$9.00/1g
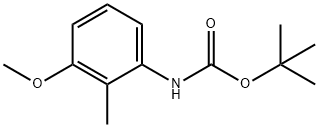
164082-77-3
13 suppliers
inquiry

19500-02-8
213 suppliers
$9.00/1g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

19500-02-8
213 suppliers
$9.00/1g