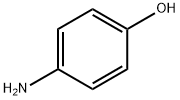
4-(Trifluoromethoxy)aniline synthesis
- Product Name:4-(Trifluoromethoxy)aniline
- CAS Number:461-82-5
- Molecular formula:C7H6F3NO
- Molecular Weight:177.12
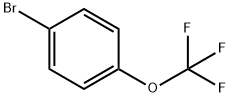
407-14-7

461-82-5
General procedure: To a flame-dried 25 mL round-bottom flask were added activated magnesium chips (7.5 mmol, 1.5 eq.) and 5 mL of anhydrous tetrahydrofuran (THF). Two drops of 1,2-dibromoethane were added to the suspension to initiate the reaction. 5 min later, a solution of p-bromotrifluoromethoxybenzene (5 mmol, 1.0 eq.) dissolved in 5 mL of anhydrous THF was slowly added to the magnesium suspension at room temperature. A mild exothermic phenomenon was observed during the reaction. After determining the concentration of Grignard's reagent by titration, 1 mmol of the reagent was transferred to another flame-dried reaction flask. The solution was diluted with 3 mL of anhydrous toluene and after cooling to the preset temperature T, a solution of oxazepane (1.2 mmol, 1.2 equiv) dissolved in 1 mL of anhydrous toluene was added. The reaction mixture was held at temperature T for a reaction time t, followed by quenching the reaction with saturated aqueous ammonium chloride (NH4Cl). (Specific reaction temperatures and reaction times need to be listed according to the actual conditions of each substrate.)
456-55-3
310 suppliers
$6.00/10g

461-82-5
461 suppliers
$10.00/1g
Yield:461-82-5 98.2%
Reaction Conditions:
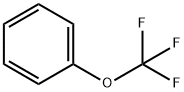
Stage #1:1-trifluoromethoxybenzene with sodium ferrate(VI);sodium bromide in dimethyl sulfoxide at 95; for 4 h;Inert atmosphere;
Stage #2: with sodium amide in dimethyl sulfoxide at 155; under 3040.2 Torr; for 10 h;Inert atmosphere;Solvent;Temperature;Reagent/catalyst;Pressure;
Steps:
1 Example 1 The method for synthesizing trifluoromethoxyaniline comprises the following steps:
1) Add 1 mol of trifluoromethoxybenzene and anhydrous DMSO to the reaction vessel under argon and vigorous stirring. Then add sodium ferrate and sodium bromide as auxiliary reaction mixture, heat to 95 ° C for 4 h, then add sodium amide, The temperature was raised to 155 ° C, the reaction pressure was raised to 4 atm, and the reaction was continued for 10 h; The ratio of trifluoromethoxybenzene to sodium amide is 1:4.5, The ratio of the amount of the trifluoromethoxybenzene to the auxiliary reaction mixture was 1:1.4. The ratio of sodium ferrate to sodium bromide is 1:1. 2) After cooling the system, pour it into 8 volumes of water. The extract was extracted with 4 times the original reaction volume of chloroform, and the extract was washed with water and dried over anhydrous sodium sulfate. Then concentrated to give the product in a molar yield of 98.2%, HPLC purity 97.7%
References:
Shandong Nong Pharmaceutical Xue Institute;Jinan Kehai Co., Ltd.;Fu Hongxin;Yang Chaohui;Wang Ling CN109134277, 2019, A Location in patent:Paragraph 0025-0049
713-65-5
236 suppliers
$8.00/1g

461-82-5
461 suppliers
$10.00/1g

407-14-7
396 suppliers
$5.00/1g

461-82-5
461 suppliers
$10.00/1g
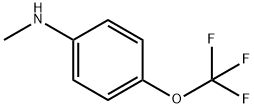
41419-59-4
80 suppliers
$32.00/1 g

461-82-5
461 suppliers
$10.00/1g