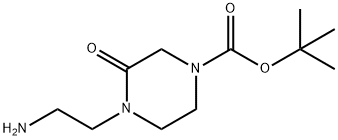
tert-butyl 4-(2-aMinoethyl)-3-oxopiperazine-1-carboxylate synthesis
- Product Name:tert-butyl 4-(2-aMinoethyl)-3-oxopiperazine-1-carboxylate
- CAS Number:234108-58-8
- Molecular formula:C11H21N3O3
- Molecular Weight:243.3

234108-59-9

234108-58-8
Step 2: A mixture of tert-butyl 4-(cyanomethyl)-3-oxopiperazine-1-carboxylate (3.0 g, 12.5 mmol) with PtO2 (300 mg, 1.32 mmol) in ethanol (20 mL) was stirred at 20 °C and reacted overnight in a hydrogen (50 psi) atmosphere. Upon completion of the reaction, the PtO2 catalyst was removed by filtration and the filtrate was concentrated under reduced pressure to afford tert-butyl 4-(2-aminoethyl)-3-oxopiperazine-1-carboxylate (2.8 g, 93% yield), the product being a yellow solid.LC-MS (ESI) analysis showed: m/z = 244.2 [M + H]+ .

234108-59-9
6 suppliers
inquiry

234108-58-8
34 suppliers
$47.00/100mg
Yield:234108-58-8 93%
Reaction Conditions:
with platinum(IV) oxide;palladium on activated charcoal;hydrogen in ethanol at 20; under 2585.81 Torr;
Steps:
20.2 tert-Butyl 4-(2-aminoethyl)-3-oxopiperazine-1-carboxylate
step 2: A mixture of tert-butyl 4-(cyanomethyl)-3-oxopiperazine-1-carboxylate (3.0 g, 12.5 mmol), PtO2 (300 mg, 1.32 mmol) in EtOH (20 mL) was stirred at 20° C. under H2 (50 psi) overnight. The Pd/C catalyst was removed by filtration, and the filtrate was concentrated under reduced pressure to afford tert-butyl 4-(2-aminoethyl)-3-oxopiperazine-1-carboxylate (2.8 g, 93%) as yellow solid. LCMS (ESI): m/z=244.2 [M+1]+.
References:
US2015/31674,2015,A1 Location in patent:Paragraph 0303; 0305; 0729
76003-29-7
245 suppliers
$5.00/5g

234108-58-8
34 suppliers
$47.00/100mg
![1-Piperazinecarboxylic acid, 4-[2-[[(1,1-dimethylethyl)dimethylsilyl]oxy]ethyl]-3-oxo-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/786683-69-0.gif)
786683-69-0
0 suppliers
inquiry

234108-58-8
34 suppliers
$47.00/100mg

910573-06-7
23 suppliers
$307.00/100mg

234108-58-8
34 suppliers
$47.00/100mg