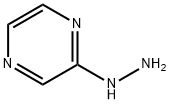
2-Hydrazinopyrazine synthesis
- Product Name:2-Hydrazinopyrazine
- CAS Number:54608-52-5
- Molecular formula:C4H6N4
- Molecular Weight:110.12

14508-49-7

54608-52-5
Step 1: Synthesis of pyrazine-2-hydrazine from 2-chloropyrazine. 2-Chloropyrazine (2.0 g, 17.5 mmol) was slowly added dropwise to 35% aqueous hydrazine hydrate (25.8 g, 97.8 mmol) at 63-65°C, and the rate of acceleration of the dropwise addition was strictly controlled so as to maintain the temperature of the reaction not exceeding 65°C. After the dropwise addition was completed, the reaction mixture was heated further up to 65°C and kept at this temperature. The reaction mixture was stirred overnight and cooled to room temperature. The solvent was removed by distillation under reduced pressure to give the product pyrazine-2-hydrazine (1.5 g, 75% yield) as a yellow powder. Mass spectrometry (ESI) analysis: calculated value C4H6N4[M]: 110.1; measured value: 111.4 [M+H]+.1H NMR (400 MHz, CD3OD) δ 8.10 (d, J=1.6 Hz, 1H), 8.00 (dd, J=3.2,1.6 Hz, 1H), 7.73 (d, J=3.2 Hz, 1H).

14508-49-7
378 suppliers
$14.00/25g

54608-52-5
235 suppliers
$9.00/1g
Yield:54608-52-5 77.8%
Reaction Conditions:
with hydrazine hydrate;sodium carbonate at 120 - 130;
Steps:
Preparation of 2-hydrazinopyrazine (8)
The mixture of 2-chloropyrazine (7) (20.0 g, 0.175 mol),hydrazine hydrate (60 mL) and sodium carbonate (27.84 g,0.264 mol) was stirred for 2 h at 120-130 °C. After completionof the reaction that was checked by TLC, the reactionmass was allowed to cool at 50-60 °C and then water(60.0 mL) followed by isopropyl alcohol (IPA) (40.0 mL)were added. The reaction mass was maintained for 16 h at 0-3 °C and then the precipitated white solid was separatedby filtration. The wet solid was dried under vacuum at 45-50 °C to obtain desired 2-hydrazinopyrazine (14.8 g, 77.8%).1H NMR (400 MHz; CD3OD), d, ppm (J, Hz): 7.94-8.14(2H, m, Ar-H); 7.69 (1H, s, Ar-H). ESI-MS m/z (rel, %):111.07 (M+H+) (100). HRMS (FAB) Calc.: C4H6N4:110.059246; Found: 111.0592 [M+H+].
References:
Mannam, Madhava Rao;Devineni, Subba Rao;Pavuluri, Chandra Mouli;Chamarthi, Naga Raju;Kottapalli, Raja Sekhara P. [Phosphorus, Sulfur and Silicon and the Related Elements,2019,vol. 194,# 9,p. 922 - 932]