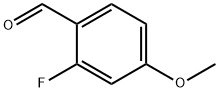
2-Fluoro-4-methoxybenzaldehyde synthesis
- Product Name:2-Fluoro-4-methoxybenzaldehyde
- CAS Number:331-64-6
- Molecular formula:C8H7FO2
- Molecular Weight:154.14
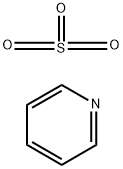
26412-87-3

405-09-4

331-64-6
The general procedure for the synthesis of 2-fluoro-4-methoxybenzaldehyde from pyridine trioxide and 2-fluoro-4-methoxybenzyl alcohol was as follows: triethylamine (0.92 mL, 6.60 mmol) was added to a solution of 2-fluoro-4-methoxybenzyl alcohol (346.9 mg, 2.22 mmol) in DMSO (8 mL). Subsequently, pyridine sulfur trioxide (1.0501 g, 6.60 mmol) was added to the mixture in batches and the change in reaction temperature was monitored. The reaction mixture was stirred for 0.5 hours. After completion of the reaction, the mixture was poured into 5% aqueous KHSO4 solution and extracted three times with ethyl acetate, the organic layers were combined and dried over anhydrous MgSO4. The organic layer was concentrated under reduced pressure and the resulting crude product was purified by silica gel column chromatography (eluent: hexane/ether=5/1) to afford the target compound 2-fluoro-4-methoxybenzaldehyde (301 mg, 88% yield). The product was characterized by 1H-NMR (CDCl3): δ 10.21 (s, 1H), 7.83 (t, 1H, J=8.3 Hz), 6.79 (ddd, 1H, J=8.3, 2.3, 0.7 Hz), 6.65 (dd, 1H, J=12.2, 2.3 Hz), 3.88 (s, 3H).

26412-87-3
458 suppliers
$21.21/50gm:

405-09-4
70 suppliers
$24.00/1g

331-64-6
256 suppliers
$14.00/1g
Yield:331-64-6 88%
Reaction Conditions:
with potassium hydrogensulfate;triethylamine in dimethyl sulfoxide;
Steps:
24.4 4
4 2-fluoro-4-methoxybenzaldehyde Triethylamine (0.92 ml, 6.60 mmol) was added to a solution of 2-fluoro-4-methoxybenzylalcohol (346.9 mg, 2.22 mmol) in DMSO (8 ml). Pyridine sulfur trioxide (1.0501 g, 6.60 mmol) was added portionwise to the mixture checking raise of the temperature. The mixture was stirred for 0.5 hours. The mixture was added to 5% KHSO4 aqueous solution, extracted three times with ethyl acetate and dried over MgSO4. The mixture was concentrated and purified by silica gel chromatography (hexane/ether=5/1) to give the title compound (301 mg; 88%). 1H-NMR(CDCl3) 10.21 (s, 1H), 7.83 (t, 1H, J =8.3 Hz), 6.79 (ddd, 1H, J=8.3, 2.3, 0.7 Hz), 6.65 (dd, 1H, J=12.2, 2.3 Hz), 3.88 (s, 3H)
References:
US6194461,2001,B1
372-20-3
395 suppliers
$7.00/5g

331-64-6
256 suppliers
$14.00/1g
114635-97-1
6 suppliers
inquiry

331-64-6
256 suppliers
$14.00/1g
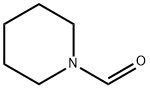
2591-86-8
299 suppliers
$14.00/5g

456-49-5
373 suppliers
$8.00/10g

331-64-6
256 suppliers
$14.00/1g
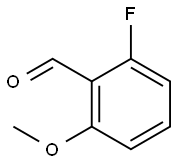
146137-74-8
194 suppliers
$6.00/250mg

