
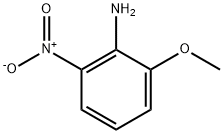
2-Chloro-3-methoxyaniline synthesis
- Product Name:2-Chloro-3-methoxyaniline
- CAS Number:113206-03-4
- Molecular formula:C7H8ClNO
- Molecular Weight:157.6
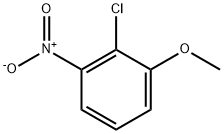
3970-39-6

113206-03-4
General procedure for the synthesis of 2-chloro-3-methoxyaniline from 2-chloro-3-nitroanisole: 2-chloro-3-nitroanisole (1.38 g, 7.36 mmol) was dissolved in a solvent mixture of glacial acetic acid (19 mL) and acetonitrile (19 mL). To this solution was added iron powder (1.64 g, 29.4 mmol). The reaction mixture was stirred under reflux conditions for 3.5 hours. After completion of the reaction, the mixture was diluted with water (70 mL) and neutralized with solid sodium carbonate (Na2CO3). Subsequently, the product was extracted with dichloromethane (3 x 150 mL). The organic phases were combined, washed with saturated saline, dried over anhydrous sodium sulfate (Na2SO4), filtered and concentrated under reduced pressure to afford the crude product 2-chloro-3-methoxyaniline (1.2 g, 100% yield) as a yellow oil. The product was used directly in the next reaction without further purification. Mass spectrometry (MS) analysis showed a molecular ion peak of 157.9 (MH)+; high performance liquid chromatography (HPLC, with TFA as additive) showed a purity of 86% at 220 nm.

3970-39-6
92 suppliers
$18.00/1g

113206-03-4
135 suppliers
$7.00/250mg
Yield: 100%
Reaction Conditions:
with iron;acetic acid in ethanol for 3.5 h;Heating / reflux;
Steps:
3C.C Step C
2-Chloro-3-nitroanisole C3 (1.38 g; 7.36 MMOL) was dissolved in a mixture of glacial acetic acid (19 ML)/ETHANOI (19 mL). To this solution was added iron powder (1.64 g; 29.4 MMOL). The mixture was stirred at reflux for 3.5 hr and worked up. The reaction mixture was diluted with water (70 mL), neutralized with solid NA2CO3 and the product extracted with CH2CI2 (3X 150 mL). The extracts were combined and washed with saturated. brine and then dried(NA2SO4), filtered and concentrated in vacuo to afford the crude product, 2-chloro-3-methoxyaniline C4 (100%; 1.2 g) as a yellow oil. This material was used as such in the following steps. MS 157.9 (MH) + ; Homogeneity by HPLC (TFA) 220 nm: 86%.
References:
BOEHRINGER INGELHEIM INTERNATIONAL GMBH;BOEHRINGER INGELHEIM PHARMA GMBH & CO KG WO2004/103996, 2004, A1 Location in patent:Page 42-43
1073118-74-7
0 suppliers
inquiry

113206-03-4
135 suppliers
$7.00/250mg
16554-45-3
140 suppliers
$16.00/1g

113206-03-4
135 suppliers
$7.00/250mg