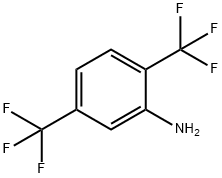
2,5-Bis(trifluoromethyl)aniline synthesis
- Product Name:2,5-Bis(trifluoromethyl)aniline
- CAS Number:328-93-8
- Molecular formula:C8H5F6N
- Molecular Weight:229.12

320-88-7

328-93-8
Example 8: To an autoclave was added 1.05 g of Raney nickel catalyst (10 wt%) and 100 mL of isopropanol followed by 10.0 g of 2,5-bis(trifluoromethyl)nitrobenzene. Hydrogen was passed under stirring until the pressure reached 5 kg/cm2 and the temperature was raised to 70 °C. The reaction was continued at 70-90°C for 8 hours. After completion of the reaction, the temperature was allowed to drop to room temperature, the hydrogen pressure was released, the catalyst was removed by filtration and the filtrate was washed with isopropanol. The filtrate was concentrated to give 6.42 g of 2,5-bis(trifluoromethyl)aniline in 73% yield and 99.4% GC purity. The structure of the product was confirmed by GC-MS, 1H-NMR and 19F-NMR analysis.

320-88-7
134 suppliers
$14.00/5g

328-93-8
314 suppliers
$10.00/10 g
Yield:328-93-8 73%
Reaction Conditions:
with hydrogen;nickel in isopropyl alcohol at 70 - 90; under 3677.86 Torr; for 8 h;
Steps:
8 EXAMPLE 8
EXAMPLE 8 Into an autoclave, 1.05 g of raney Nickel catalyst (10 wt%) and 100 ml of isopropanol were introduced, and 10.0 g of 2,5-bis(trifluoromethyl)nitrobenzene was added thereto in small amounts.. hydrogen gas was blown until 5 kg/cm2 with stirring, and the temperature was increased to 70°C. Reaction was carried out at from 70 to 90°C for 8 hours.. After the temperature was recovered to room temperature, hydrogen gas was evacuated, and the raney Nickel was subjected to filtration and washed with isopropanol.. The solvent was concentrated to obtain 2,5-bis(trifluoromethyl)aniline (6.42 g, yield 73%, purity by GC 99.4%).. As a result of analysis by GC-Mass, 1H-NMR and 19F-NMR, the product was confirmed to be 2,5-bis(trifluoromethyl)aniline.
References:
ASAHI GLASS COMPANY LTD. EP1468983, 2004, A1 Location in patent:Page 6
433-19-2
291 suppliers
$26.00/25g

328-93-8
314 suppliers
$10.00/10 g
328-91-6
31 suppliers
$30.00/1g

328-93-8
314 suppliers
$10.00/10 g
![2-Pyridinecarboxamide, N-[3-(trifluoromethyl)phenyl]-](/CAS/20210305/GIF/61350-01-4.gif)
61350-01-4
0 suppliers
inquiry

328-93-8
314 suppliers
$10.00/10 g
10388-10-0
12 suppliers
inquiry

328-93-8
314 suppliers
$10.00/10 g