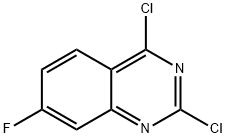
2,4-Dichloro-7-fluoroquinazoline synthesis
- Product Name:2,4-Dichloro-7-fluoroquinazoline
- CAS Number:174566-15-5
- Molecular formula:C8H3Cl2FN2
- Molecular Weight:217.03

76088-98-7

174566-15-5
General procedure for the synthesis of 2,4-dichloro-7-fluoroquinazoline from 7-fluoroquinazoline-2,4(1H,3H)-dione: 7-fluoroquinazoline-2,4(1H,3H)-dione (5.26 g, 29.2 mmol) was mixed with phosphorus trichloride (85 mL) and stirred for 3 days under reflux conditions. Upon completion of the reaction, the mixture was cooled to room temperature and slowly poured into ice water. The resulting solid product was collected by filtration and dried under vacuum to afford the title compound 2,4-dichloro-7-fluoroquinazoline (3.82 g) as a yellow solid. The structure of the product was confirmed by 1H NMR (400 MHz, CDCl3): δ 8.32 (m, 1H), 7.63 (d, 1H), 7.49 (t, 1H).

76088-98-7
82 suppliers
$45.00/50mg

174566-15-5
139 suppliers
$25.00/100mg
Yield: 78%
Reaction Conditions:
with trichlorophosphate for 72 h;Heating / reflux;
Steps:
100
A suspensionof 7-fluoro-2, 4-dioxo(1H, 3X) quinazoline (Method 98,1. 8 g, 10 mmol) inPOC13 (30 ml) was heated under reflux for 72 hours. The brown coloured solution was concentrated to dryness under vacuum. The residue was treated with ice water (50 ml) and filtered. The residue was washed with ice-cold water (10 ml) and dried to give the desired product(1. 7 g, 78%). 1H NMR(CDC13)5 7.92(m,1 H), 7.95 (d, J = 2.9 Hz, 1 H), 8.42(m,1 H). MS: m/z 219 (M+3), 217(M+1).
References:
ASTRAZENECA AB;ASTRAZENECA UK LIMITED WO2005/49033, 2005, A1 Location in patent:Page/Page column 113

76088-98-7
82 suppliers
$45.00/50mg

174566-15-5
139 suppliers
$25.00/100mg
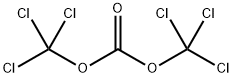
32315-10-9
437 suppliers
$10.00/1g

80517-22-2
131 suppliers
inquiry

174566-15-5
139 suppliers
$25.00/100mg
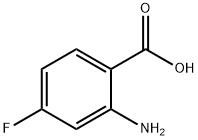
446-32-2
449 suppliers
$6.00/5g

174566-15-5
139 suppliers
$25.00/100mg