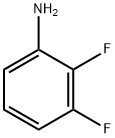
2,3-Difluoroaniline synthesis
- Product Name:2,3-Difluoroaniline
- CAS Number:4519-40-8
- Molecular formula:C6H5F2N
- Molecular Weight:129.11
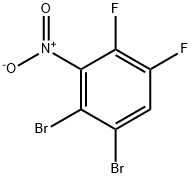
1481-57-8

4519-40-8
The general procedure for the synthesis of 2,3-difluoroaniline from 2,3-dibromo-5,6-difluoronitrobenzene was as follows: first, 0.638 g (2.01 mmol) of 2,3-dibromo-5,6-difluoronitrobenzene was dissolved in 4 mL of methanol. Subsequently, 48.4 mg of 10% Pd/C (50% wet) catalyst and 6.2 mg (0.06 mmol) of triethylamine were added to the solution. The atmosphere in the reaction vessel was replaced with hydrogen and the reaction mixture was stirred at 50 °C for 2 h under slight hydrogen pressure. The reaction mixture was analyzed by HPLC, which showed the presence of 2.2 area% of 2,3-dibromo-5,6-difluoroaniline as a residual intermediate. The reaction was continued under the same conditions for 0.5 hours. After completion of the reaction, the catalyst was removed by filtration. The resulting liquid was quantitatively analyzed by HPLC and the yield of 2,3-difluoroaniline was measured to be 93% and the intermediate 2,3-dibromo-5,6-difluoroaniline was completely consumed.

1481-57-8
46 suppliers
$128.00/1g

4519-40-8
300 suppliers
$10.00/1g
Yield:4519-40-8 93%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen;triethylamine in methanol at 50; for 2.5 h;Temperature;
Steps:
16 Example 16 (Production of 2,3-difluoroaniline)
First, 0.638 g (2.01 mmol) of 1,2-dibromo-4,5-difluoro-3-nitrobenzene was dissolved in 4 mL of methanol, and then 48.4 mg of 10% Pd/C (50% wet) and 6.2 mg (0.06 mmol) of triethylamine were added. The atmosphere inside the reaction vessel was substituted with hydrogen, and the reaction mixture was reacted at 50°C for 2 hours under slight hydrogen pressurization. Analysis of the resulting reaction product by HPLC revealed 2.2 area% of 2,3-dibromo-5,6-difluoroaniline as a residual intermediate. Reaction was continued under the same conditions for a further 0.5 hours. The catalyst was then removed by filtration. The resulting liquid was subjected to quantitative analysis by HPLC. The yield of 2,3-difluoroaniline was 93%. The intermediate 2,3-dibromo-5,6-difluoroaniline had been eliminated.
References:
EP2940002,2015,A1 Location in patent:Paragraph 0075

771-69-7
345 suppliers
$5.00/10g

4519-40-8
300 suppliers
$10.00/1g
87-61-6
230 suppliers
$14.00/25g

4519-40-8
300 suppliers
$10.00/1g
5509-65-9
504 suppliers
$10.00/10g
367-11-3
319 suppliers
$10.00/1g

4519-40-8
300 suppliers
$10.00/1g
64695-78-9
228 suppliers
$8.00/5g

4519-40-8
300 suppliers
$10.00/1g