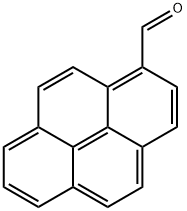
1-Pyrenecarboxaldehyde synthesis
- Product Name:1-Pyrenecarboxaldehyde
- CAS Number:3029-19-4
- Molecular formula:C17H10O
- Molecular Weight:230.26
24463-15-8

3029-19-4
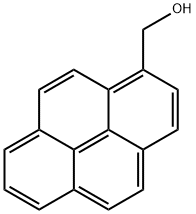
General procedure for the synthesis of 1-pyrenecarboxaldehyde from 1-pyrenemethanol: To a round-bottom flask were added 1-pyrenemethanol (2 mmol), copper trifluoromethanesulfonate (CuOTf, 0.1 mmol, 0.05 eq.), (S)-5-(pyrrolidin-2-yl)-1H-tetrazole (0.1 mmol, 0.05 eq.), 2,2,6,6-tetramethylpiperidine-1-yloxy ( TEMPO, 0.1 mmol, 0.05 eq.), 4-dimethylaminopyridine (DMAP, 0.15 mmol, 0.075 eq.) and acetonitrile (CH3CN, 5 mL). The reaction mixture was stirred and exposed to air at 25 °C until the reaction was completed, and the progress of the reaction was monitored by thin layer chromatography (TLC). After completion of the reaction, the acetonitrile was removed by evaporation under vacuum. The residue was diluted with dichloromethane (CH2Cl2, 5 mL) and filtered through a silica gel plug to obtain the target product 1-pyrenecarboxaldehyde.
Yield:3029-19-4 96%
Reaction Conditions:
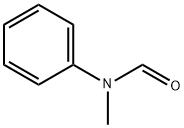
with titanium tetrachloride in dichloromethane at 0 - 20; for 2.5 h;
Steps:
Synthesis of 1-hydroxypyrene (3)
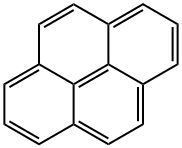
To a stirred CH2Cl2 (90 mL) solution of pyrene (2.022 g, 10 mmol) was added Cl2CHOCH3 (1.2 mL, 13 mmol) at 0 °C. To the solution was slowly added TiCl4 (2.0 mL, 18 mmol). The color of solution turned from yellow to purplish red. The mixture was stirred at 0 °C for 1 h and at room temperature for 1.5 h. The solution was poured into cold water (100 mL), and stirred until the color turned from purplish red to yellow. The mixture was extracted with CH2Cl2 (50 mL x 2). The combined organic layers were washed with brine (50 mL), separated, dried over MgSO4, filtered, and concentrated in vacuo to give 1-formylpyrene (2.24 g, 96% yield). Yellow solid; 1H NMR (400 Mz, CDCl3) d 8.08-8.13 (m, 2H), 8.23-8.36 (m, 5H), 8.47 (d, J = 8.0 Hz, 1H), 9.45 (d, J = 9.6 Hz, 1H), 10.80 (s, 1H) ppm. To a stirred CH2Cl2 (20 mL) solution of 1-formylpyrene (0.46 g, 2 mmol) was added m-CPBA (0.68 g, 3 mmol), and the resulting solution was stirred at reflux for 24 h, then concentrated in vacuo. To the residue was added THF (5 mL), MeOH (5 mL), and 25% KOH aq (0.6 mL), giving a mixture that was stirred at room temperature for 4 h, then concentrated in vacuo. The residue was dissolved in 2% KOH aq (10 mL). The solution was extracted with CH2Cl2 (10 mL x 2). The combined organic layers were washed with ice-cooled HCl aq (pH = 2), separated, dried over MgSO4, filtered, and concentrated in vacuo, giving as residue that was subjected to silica gel column chromatography (eluent; CHCl3, Rf = 0.3) to give 1-hydroxypyrene (3, 0.223 g, 50% yield). Gray solid; 1H NMR (400 Mz, CDCl3) d 5.70 (brs, 1H), 7.48 (d, J = 6.4 Hz, 1H), 7.91 (d, J = 8.8 Hz, 1H), 7.96-7.99 (m, 2H), 8.03-8.07 (m, 2H), 8.10-8.13 (m, 2H), 8.35 (d, J = 7.6 Hz, 1H).
References:
Maeda, Hajime;Akai, Tomomi;Segi, Masahito [Tetrahedron Letters,2017,vol. 58,# 46,p. 4377 - 4380] Location in patent:supporting information

24463-15-8
118 suppliers
$20.00/10mg

3029-19-4
202 suppliers
$15.00/1g
2381-21-7
97 suppliers
$115.00/100mg

3029-19-4
202 suppliers
$15.00/1g

164461-18-1
250 suppliers
$6.00/250mg

298-12-4
473 suppliers
$5.00/25g

3029-19-4
202 suppliers
$15.00/1g