
1-Pyrenylboronic acid synthesis
- Product Name:1-Pyrenylboronic acid
- CAS Number:164461-18-1
- Molecular formula:C16H11BO2
- Molecular Weight:246.07
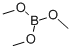
121-43-7
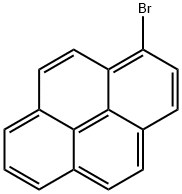
1714-29-0

164461-18-1
The general procedure for the synthesis of 1-pyrenylboronic acid from trimethyl borate and 1-bromopyrene was as follows: 1-bromopyrene (30 g, 107 mmol) was added to a round-bottom flask and dissolved in 240 mL of tetrahydrofuran (THF). Under cooling conditions, n-BuLi (1.6 M, 80 mL) in hexane was slowly added dropwise, keeping the reaction temperature at -78 °C. After the dropwise addition was completed, the reaction mixture continued to be stirred at -78 °C for about 1 hour. Subsequently, trimethyl borate (15.5 g, 149 mmol) was added and stirring was continued at -78 °C for 1 hour. After that, the reaction mixture was warmed up to room temperature and stirred for about 2 hours. After completion of the reaction, the reaction solution was adjusted to acidic with 2N HCl. Extraction was carried out with ethyl acetate (EA) and hexane, and the organic phases were combined and concentrated under reduced pressure to remove the solvent to give 19.5 g of 1-pyrenylboronic acid in 74% yield.
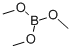
121-43-7
385 suppliers
$13.00/25mL

1714-29-0
308 suppliers
$6.00/1g

164461-18-1
250 suppliers
$6.00/250mg
Yield:164461-18-1 74%
Reaction Conditions:
Stage #1:1-bromopyrene with n-butyllithium in tetrahydrofuran;hexane at -78; for 1 h;
Stage #2:Trimethyl borate in tetrahydrofuran;hexane at -78 - 20; for 2 h;
Steps:
8-1 Synthesis Example 8-1. 1-pyreneboronic acid synthesis
To a round bottom flask1-Bromo-pyrene (30g, 107 mmol) was dissolved in 240 mL THF. In the cooling state up to -78 n-BuLi in n-hexane (1.6M) was put in a slow (80 mL). At the end of the addition and then stirred for about 1 hour at -78 was put trimethyl borate (15.5 g, 149 mmol). After 1 hour stirring at -78 stirred at room temperature for about two hours and put in a 2N HCl was adjusted to acidic. While the removal of the solvent after extraction with EA obtained with hexane to give the 1-pyreneboronic acid 19.5 g (yield 74 %).
References:
SFC Co., Ltd.;Yu, Say Jin;Lee, Sang Hae;Sim, So Young KR101515814, 2015, B1 Location in patent:Paragraph 0343; 0344
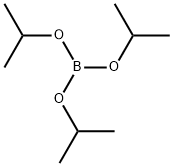
5419-55-6
392 suppliers
$12.00/5g

1714-29-0
308 suppliers
$6.00/1g

164461-18-1
250 suppliers
$6.00/250mg

1714-29-0
308 suppliers
$6.00/1g

164461-18-1
250 suppliers
$6.00/250mg
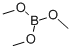
121-43-7
385 suppliers
$13.00/25mL

1714-29-0
308 suppliers
$6.00/1g
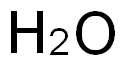
7732-18-5
499 suppliers
$12.69/100ml

164461-18-1
250 suppliers
$6.00/250mg
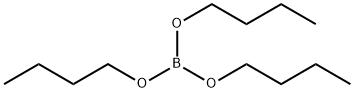
688-74-4
333 suppliers
$10.40/25mL

1714-29-0
308 suppliers
$6.00/1g

164461-18-1
250 suppliers
$6.00/250mg