
1-METHYL-1H-BENZIMIDAZOLE-2-CARBOXYLIC ACID synthesis
- Product Name:1-METHYL-1H-BENZIMIDAZOLE-2-CARBOXYLIC ACID
- CAS Number:20572-01-4
- Molecular formula:C9H8N2O2
- Molecular Weight:176.17
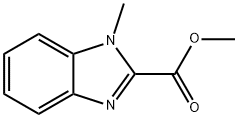
2849-92-5

20572-01-4
1. Methyl 1-methyl-1H-benzimidazole-2-carboxylate (190 mg, 1.0 mmol) and NaOH (80 mg, 2.0 mmol) in a mixture of MeOH/H2O (1:1, 20 mL) were stirred and reacted for 1 hr at 50°C. The reaction was carried out with the help of a stirring device. 2. Upon completion of the reaction, the mixture was concentrated and then diluted with 15 mL of water. 3. The pH of the diluted solution was adjusted to 2 with concentrated hydrochloric acid, at which time a precipitate was formed. 4. 4. The precipitate was collected by filtration, washed with water and dried to give 1-methyl-1H-benzimidazole-2-carboxylic acid (176 mg, 1.0 mmol, 100% yield). 5. The product was analyzed by ESI-MS and the (M + 1) peak was 177, which corresponded to the calculated value of C9H8N2O2 (176).
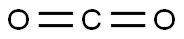
124-38-9
133 suppliers
$175.00/23402

20572-01-4
84 suppliers
$45.00/10mg
Yield: 72%
Reaction Conditions:
Stage #1:1-Methylbenzimidazole with n-butyllithium in diethyl ether;hexane at -78 - -60; for 0.5 h;
Stage #2:carbon dioxide with hydrogenchloride in diethyl ether;hexane at -78 - -5; for 1.16667 h;
Steps:
375.A EXAMPLE 375;N-(4-{4-amino-7-[(1E)-3-(diethylamino)-1-propenyl]thieno[3,2-c]pyridin-3-yl}-2-methoxyphenyl)-1-methyl-1H-benzimidazole-2-carboxamide; EXAMPLE 375A; 1-methyl-1H-benzimidazole-2-carboxylic acid
A suspension of 1-methyl-1H-benzimidazole (5.0 g, 37.83 mmol) in diethyl ether at -78 C. was treated slowly with 1.6M n-butyllithium in hexanes (26 mL, 41.61 mmol) while maintaining the temperature at below -60 C., and stirred at -78 C. for 30 minutes. Carbon dioxide was bubbled through the reaction solution for 40 minutes. The dry ice bath was then removed to bring the temperature to -5 C. Concentrated hydrochloric acid (7 mL) was added slowly. The reaction mixture was stirred at -5 C. for 30 minutes, and then water (10 mL) was added. The solid was collected by filtration and dried to remove the excess water to provide 4.8 g (72%) of the desired product which was directly used in the next reaction without further purification or analysis.
References:
Betschmann, Patrick;Burchat, Andrew;Calderwood, David;Curtin, Michael L.;Davidsen, Steven K.;Davis, Heather M.;Frey, Robin R.;Heyman, Howard R.;Hirst, Gavin;Hrnciar, Peter;Michaelides, Michael;Rafferty, Paul US2005/20619, 2005, A1 Location in patent:Page 68

2849-92-5
52 suppliers
$89.00/100mg

20572-01-4
84 suppliers
$45.00/10mg

1632-83-3
188 suppliers
$6.00/1g

20572-01-4
84 suppliers
$45.00/10mg

1632-83-3
188 suppliers
$6.00/1g
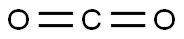
124-38-9
133 suppliers
$175.00/23402

20572-01-4
84 suppliers
$45.00/10mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

20572-01-4
84 suppliers
$45.00/10mg