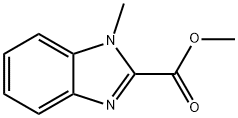
1H-Benzimidazole-2-carboxylicacid,1-methyl-,methylester(9CI) synthesis
- Product Name:1H-Benzimidazole-2-carboxylicacid,1-methyl-,methylester(9CI)
- CAS Number:2849-92-5
- Molecular formula:C10H10N2O2
- Molecular Weight:190.2

5805-53-8

74-88-4

2849-92-5
To a solution of 1H-benzimidazole-2-carboxylic acid methyl ester (177 mg, 1.0 mmol) in anhydrous N,N-dimethylformamide (DMF, 5 mL) was added sodium hydride (60% dispersed in mineral oil, 62 mg, 1.5 mmol) at 0 °C under nitrogen protection. The reaction mixture was stirred at 0 °C for 0.5 h. After that, iodomethane (284 mg, 2.0 mmol) was slowly added. Subsequently, the reaction mixture was continued to be stirred at room temperature for 4 hours. After completion of the reaction, the mixture was diluted with saturated saline at 0 °C and extracted with ethyl acetate (3 x 20 mL). The combined organic phases were washed sequentially with water (2 x 15 mL) and saturated saline (20 mL), dried over anhydrous sodium sulfate and filtered. The filtrate was concentrated under reduced pressure and purified by fast column chromatography to afford methyl 1-methyl-1H-benzimidazole-2-carboxylate (180 mg, 90% yield) as a light yellow solid.ESI-MS (M + 1): 191 (calculated value: C10H10N2O2).

5805-53-8
71 suppliers
$50.00/250mg

74-88-4
359 suppliers
$15.00/10g

2849-92-5
52 suppliers
$89.00/100mg
Yield:2849-92-5 90%
Reaction Conditions:
Stage #1: BICA methyl esterwith sodium hydride in N,N-dimethyl-formamide;mineral oil at 0; for 0.5 h;Inert atmosphere;
Stage #2: methyl iodide in N,N-dimethyl-formamide;mineral oil at 20; for 4 h;
Steps:
25.1
To a solution of lH-benzoimidazole-2-carboxylic acid methyl ester (18) (177 mg, 1.0 mmol) in dry DMF (5 mL) was added sodium hydride (applied as 60%> dispersion in oil, 62 mg, 1.5 mmol)) at 0°C under N2 atmosphere. After 0.5 h, iodomethane (284 mg, 2.0 mmol) was added slowly. The reaction mixture was stirred at RT for 4 h. The reaction was diluted with brine at 0°C and extracted with EtOAc (3 x 20 mL). The combined organics were washed with water (2 x 15 mL), brine (20 mL), dried over sodium sulfate and filtered. The filtrate was concentrated and purified by flash column chromatography to provide the title compound (81) (180 mg, 90% yield) as a light yellow solid.ESI-MS (M+l): 191 calc. for C10H10
References:
WO2011/143365,2011,A1 Location in patent:Page/Page column 170-171

1632-83-3
188 suppliers
$6.00/1g
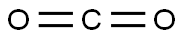
124-38-9
134 suppliers
$214.00/14L

74-88-4
359 suppliers
$15.00/10g

2849-92-5
52 suppliers
$89.00/100mg
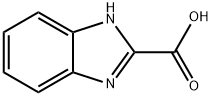
2849-93-6
188 suppliers
$6.00/250mg

74-88-4
359 suppliers
$15.00/10g

2849-92-5
52 suppliers
$89.00/100mg
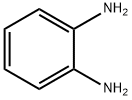
95-54-5
537 suppliers
$9.00/1g

2849-92-5
52 suppliers
$89.00/100mg

