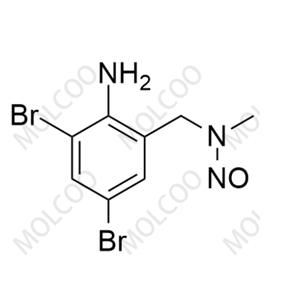
Bromhexine Nitroso Impurity 34 NEW
| Price | Get Latest Price | ||
| Package | 10mg | 50mg | 100mg |
| Min. Order: | 10mg |
| Supply Ability: | 1000 |
| Update Time: | 2025-07-17 |
Product Details
| Product Name: Bromhexine Nitroso Impurity 34 | Min. Order: 10mg |
| Purity: 99%+ HPLC | Supply Ability: 1000 |
| Release date: 2025/07/17 | |
| Molecular formula: C8H9Br2N3O |
Bromhexine Nitroso Impurity 34

WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com.
Product Number: B008034
English Name: Bromhexine Nitroso Impurity 34
English Alias: N-(2-amino-3,5-dibromobenzyl)-N-methylnitrous amide
CAS Number: None
Molecular Formula: C?H?Br?N?O
Molecular Weight: 322.98
Well-defined structure and high stability, which can be used to analyze the by-product formation mechanism of nitrosation reactions during bromhexine synthesis or storage, optimizing processes to control nitroso impurity generation;
Serving as a reference standard containing nitroso, amino, and dibromophenyl structures, providing a standard substance for detecting nitroso impurities in drugs, and helping to evaluate drug safety (nitroso compounds may have potential carcinogenicity);
Helping study the impact of nitroso structures on drug stability and toxicological properties to provide a scientific basis for formulating impurity control strategies.
Drug Development: Used as an impurity reference standard to identify and quantify N-nitroso impurities in bromhexine preparations, evaluating the purity of APIs and formulations;
Quality Control: Acting as a standard substance to validate the sensitivity of detection methods (e.g., HPLC or LC-MS), ensuring nitroso impurity content meets ICH guideline requirements during production;
Toxicological Research: Assisting in evaluating the potential genotoxicity of nitroso impurities to provide data support for drug safety evaluation.
Synthesis Methods: Developing high-purity synthesis processes for Bromhexine Nitroso Impurity 34, solving the challenge of poor stability of nitroso compounds to meet the needs of toxicological research and quality control;
Detection Technologies: Establishing trace detection methods for nitroso impurities (detection limits reach ppb level) using ultra-high-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) and other technologies;
Toxicological Evaluation: Studying the potential mutagenicity and carcinogenicity of this impurity through in vitro Ames tests and animal models;
Process Control: Analyzing the inducements (such as raw material residues, reaction conditions) of nitrosation reactions to optimize the synthesis route or storage conditions to reduce the generation of nitroso impurities
Product Number: B008034
English Name: Bromhexine Nitroso Impurity 34
English Alias: N-(2-amino-3,5-dibromobenzyl)-N-methylnitrous amide
CAS Number: None
Molecular Formula: C?H?Br?N?O
Molecular Weight: 322.98
Well-defined structure and high stability, which can be used to analyze the by-product formation mechanism of nitrosation reactions during bromhexine synthesis or storage, optimizing processes to control nitroso impurity generation;
Serving as a reference standard containing nitroso, amino, and dibromophenyl structures, providing a standard substance for detecting nitroso impurities in drugs, and helping to evaluate drug safety (nitroso compounds may have potential carcinogenicity);
Helping study the impact of nitroso structures on drug stability and toxicological properties to provide a scientific basis for formulating impurity control strategies.
Drug Development: Used as an impurity reference standard to identify and quantify N-nitroso impurities in bromhexine preparations, evaluating the purity of APIs and formulations;
Quality Control: Acting as a standard substance to validate the sensitivity of detection methods (e.g., HPLC or LC-MS), ensuring nitroso impurity content meets ICH guideline requirements during production;
Toxicological Research: Assisting in evaluating the potential genotoxicity of nitroso impurities to provide data support for drug safety evaluation.
Synthesis Methods: Developing high-purity synthesis processes for Bromhexine Nitroso Impurity 34, solving the challenge of poor stability of nitroso compounds to meet the needs of toxicological research and quality control;
Detection Technologies: Establishing trace detection methods for nitroso impurities (detection limits reach ppb level) using ultra-high-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) and other technologies;
Toxicological Evaluation: Studying the potential mutagenicity and carcinogenicity of this impurity through in vitro Ames tests and animal models;
Process Control: Analyzing the inducements (such as raw material residues, reaction conditions) of nitrosation reactions to optimize the synthesis route or storage conditions to reduce the generation of nitroso impurities
This product is intended for laboratory use only!
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/10mg |
VIP2Y
|
ShenZhen H&D Pharmaceutical Technology Co., LTD
|
2025-03-31 | |
| $0.00/5mg |
VIP1Y
|
Guangzhou Tosun Pharmaceutical Ltd
|
2025-01-23 | |
| $0.00/1kg |
VIP2Y
|
Shaanxi TNJONE Pharmaceutical Co., Ltd
|
2024-04-29 |








 China
China