|
|
| | P-NITROPHENYL 6-DEOXY-ALPHA-L-MANNOPYRANOSIDE Basic information |
| Product Name: | P-NITROPHENYL 6-DEOXY-ALPHA-L-MANNOPYRANOSIDE | | Synonyms: | 3-Nitrophenyl ?L-rhamnopyranoside;p-Nitrophenyl alpha-L-rhamnoside;3-Nitrophenyl a-L-rhaMnopyranoside;p-nitrophenyl α-L-Rhap;3-Nitrophenyl alpha-L-rhamnopyranoside;P-NITROPHENYL-ALPHA-RHAMNOSIDE;P-NITROPHENYL ALPHA-L-RHAMNOPYRANOSIDE;P-NITROPHENYL 6-DEOXY-ALPHA-L-MANNOPYRANOSIDE | | CAS: | 18918-31-5 | | MF: | C12H15NO7 | | MW: | 285.25 | | EINECS: | 1592732-453-0 | | Product Categories: | | | Mol File: | 18918-31-5.mol | 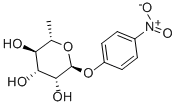 |
| | P-NITROPHENYL 6-DEOXY-ALPHA-L-MANNOPYRANOSIDE Chemical Properties |
| Melting point | 179°C | | Boiling point | 515.4±50.0 °C(Predicted) | | density | 1.503±0.06 g/cm3(Predicted) | | storage temp. | −20°C | | solubility | DMF: 20 mg/ml; DMSO: 15 mg/ml; Ethanol: 0.25 mg/ml; PBS (pH 7.2): 2 mg/ml | | pka | 12.68±0.70(Predicted) | | form | powder | | color | Off-white to light yellow | | InChI | InChI=1/C12H15NO7/c1-6-9(14)10(15)11(16)12(19-6)20-8-4-2-7(3-5-8)13(17)18/h2-6,9-12,14-16H,1H3/t6-,9-,10+,11+,12-/s3 | | InChIKey | YILIDCGSXCGACV-KOILSSBMNA-N | | SMILES | [C@H]1(O[C@H]([C@H](O)[C@@H](O)[C@H]1O)C)OC1=CC=C([N+]([O-])=O)C=C1 |&1:0,2,3,5,7,r| | | LogP | 1.170 (est) |
| WGK Germany | 3 | | HS Code | 29400090 |
| | P-NITROPHENYL 6-DEOXY-ALPHA-L-MANNOPYRANOSIDE Usage And Synthesis |
| Uses | 4-Nitrophenyl α-L-Rhamnopyranoside is a chromogenic substrate for naringinase. | | Uses | 4-Nitrophenyl α-L-rhamnopyranoside has been used as a substrate to determine β-glucosidase activity. It has also been used as a substrate to measure α-L-rhamnosidase activity of Oenococcus oeni and Aspergillus terreus. | | Biochem/physiol Actions | 4-Nitrophenyl α-L-rhamnopyranoside is a substrate for determining α-L-rhamnosidase activity. The hydrolysis of 4-Nitrophenyl α-L-rhamnopyranoside by the enzyme yields 4-nitrophenol, measured at 405 nm spectrophotometrically. |
| | P-NITROPHENYL 6-DEOXY-ALPHA-L-MANNOPYRANOSIDE Preparation Products And Raw materials |
|






