|
|
| | 3-AMINO-3-(4-CHLORO-PHENYL)-PROPAN-1-OL Basic information |
| Product Name: | 3-AMINO-3-(4-CHLORO-PHENYL)-PROPAN-1-OL | | Synonyms: | RARECHEM AK ML 0281;3-AMINO-3-(4-CHLORO-PHENYL)-PROPAN-1-OL;DL-beta-(4-chlorophenyl)alaninol;REF DUPL: DL-beta-(4-chlorophenyl)alaninol;3-AMino-3-(4-chlorophenyl)-1-propanol;3-(4-chlorophenyl)-3-aMino-1-propanol;gamma-Amino-4-chlorobenzenepropanol;Benzenepropanol, .gamma.-amino-4-chloro- | | CAS: | 68208-26-4 | | MF: | C9H12ClNO | | MW: | 185.65 | | EINECS: | | | Product Categories: | pharmacetical | | Mol File: | 68208-26-4.mol | 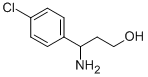 |
| | 3-AMINO-3-(4-CHLORO-PHENYL)-PROPAN-1-OL Chemical Properties |
| storage temp. | under inert gas (nitrogen or Argon) at 2–8 °C | | InChI | InChI=1S/C9H12ClNO/c10-8-3-1-7(2-4-8)9(11)5-6-12/h1-4,9,12H,5-6,11H2 | | InChIKey | JGNACDMQJLVKIU-UHFFFAOYSA-N | | SMILES | C1(C=CC(Cl)=CC=1)C(N)CCO |
| | 3-AMINO-3-(4-CHLORO-PHENYL)-PROPAN-1-OL Usage And Synthesis |
| Uses | 3-Amino-3-(4-chlorophenyl)-1-propanol is a useful reactant for the synthesis of meta-?functionalized pyridines. | | Synthesis | General procedure for the synthesis of 3-amino-3-(4-chlorophenyl)propan-1-ol from 3-amino-3-(4-chlorophenyl)propionic acid: preparation of intermediate 29: 3-amino-3-(4-chlorophenyl)propionic acid (2.50 g, 12.52 mmol) was suspended in THF (75 mL) under nitrogen protection and cooled to 0 °C. To this suspension was added 3-amino-3-(4-chlorophenyl)propan-1-ol borane-tetrahydrofuran complex (94.0 mL, 93.92 mmol) dropwise over 20 min. After the dropwise addition was completed, the reaction mixture was stirred at 0°C for 30 minutes, followed by warming to 22°C and continued stirring for 5 hours. Upon completion of the reaction, the reaction mixture was added to methanol (500 mL) in batches. The mixture was concentrated, redissolved in methanol (250 mL) and concentrated again (this process was repeated three times). The residue was dissolved in DCM (200 mL) and washed with 1N NaOH (150 mL). The aqueous layer was back-extracted with DCM (5 x 100 mL) and all organic layers were combined. The combined organic layers were washed with saturated brine (2 × 150 mL), dried over MgSO4 and concentrated to give a white semi-solid. The crude product was purified by fast silica gel chromatography with an elution gradient of 5% to 7% (10:1 MeOH/concentrated NH3 (aqueous solution)) of DCM solution. The pure grades were collected and evaporated to dryness to afford 3-amino-3-(4-chlorophenyl)propan-1-ol (1.320 g, 56.8% yield) as a white solid.1H NMR (399.902 MHz, CDCl3) δ 1.87 (2H, m), 2.34 (2H, br.s), 3.79 (2H, m), 4.13 (1H, t), 7.24 (2H, d), 7.32 (2H, d).MS m/e MH+ 169. | | References | [1] Journal of Medicinal Chemistry, 2013, vol. 56, # 5, p. 2059 - 2073
[2] Patent: WO2009/47563, 2009, A1. Location in patent: Page/Page column 102-103 |
| | 3-AMINO-3-(4-CHLORO-PHENYL)-PROPAN-1-OL Preparation Products And Raw materials |
|