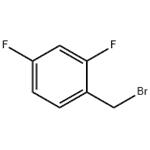
- 2,4-Difluorobenzyl bromide
-
- $100.00 / 1KG
-
2023-12-26
- CAS:23915-07-3
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
|
| | 2,4-Difluorobenzyl bromide Basic information |
| Product Name: | 2,4-Difluorobenzyl bromide | | Synonyms: | 4-Difluorobenzyl broMide;alpha-BroMo-2,4-difluorotoluene, 98% 5GR;ALPHA-BROMO-2,4-DIFLUOROTOLUENE;Toluene, α-broMo-2,4-difluoro- (8CI);1-(Bromomethyl)-2,4-difluorobenzene, alpha-Bromo-2,4-difluorotoluene;2,4-Difluorobenzyl broMide 98%, 4-Difluorobenzyl broMide 98%;2,4-DIFLUOROBENZYL BROMIDE;TIMTEC-BB SBB006567 | | CAS: | 23915-07-3 | | MF: | C7H5BrF2 | | MW: | 207.02 | | EINECS: | 245-938-3 | | Product Categories: | Aromatic Halides (substituted);Benzene series;Miscellaneous;Fluorinated benzene series;Fluoro-contained benzyl bromide series;Fluorine series | | Mol File: | 23915-07-3.mol | 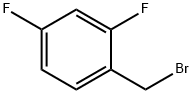 |
| | 2,4-Difluorobenzyl bromide Chemical Properties |
| Melting point | 18 °C | | Boiling point | 28 °C | | density | 1.613 g/mL at 25 °C(lit.) | | refractive index | n20/D 1.525(lit.) | | Fp | 104 °F | | storage temp. | Inert atmosphere,2-8°C | | form | Liquid | | Specific Gravity | 1.63 | | color | Clear yellow | | Sensitive | Lachrymatory | | BRN | 4177539 | | CAS DataBase Reference | 23915-07-3(CAS DataBase Reference) |
| Hazard Codes | C,F | | Risk Statements | 10-34-42/43-36 | | Safety Statements | 23-26-36/37/39-45-25-16 | | RIDADR | UN 2920 8/PG 2 | | WGK Germany | 3 | | Hazard Note | Corrosive/Lachrymatory | | HazardClass | 8 | | PackingGroup | III | | HS Code | 29039990 |
| | 2,4-Difluorobenzyl bromide Usage And Synthesis |
| Chemical Properties | CLEAR YELLOW LIQUID | | Uses | 2,4-Difluorobenzyl bromide has been used in the preparation of:
- novel 1,2,4-triazolium derivatives
- 1,5-biaryl pyrrole EP1 receptor antagonists
| | Synthesis | The general procedure for the synthesis of 2,4-difluorobenzyl bromide from 2,4-difluorobenzyl alcohol was as follows: to a solution of diethyl ether (10 mL) of 2,4-difluorobenzyl alcohol (450 mg, 3.12 mmol) was slowly added phosphorus tribromide (0.2 mL, 2.18 mmol) at 0 °C. The reaction mixture was stirred at room temperature for 2 hours. Upon completion of the reaction (monitored by thin layer chromatography), the reaction was quenched with ice water (20 mL) and extracted with ethyl acetate (2 x 20 mL). The organic layers were combined, washed sequentially with water (40 mL) and saturated saline (40 mL), dried over anhydrous sodium sulfate, and concentrated under reduced pressure to give the crude product. Purification by silica gel column chromatography with 5% ethyl acetate/hexane as eluent gave 2,4-difluorobenzyl bromide (420 mg, 2.02 mmol, 65% yield) as a colorless liquid.1H NMR (200 MHz, CDCl3): δ 7.43-7.31 (m, 1H), 6.92-6.77 (m, 2H), 4.48 (s, 2H). | | References | [1] Patent: US2012/329788, 2012, A1. Location in patent: Page/Page column 26 |
| | 2,4-Difluorobenzyl bromide Preparation Products And Raw materials |
|