|
|
| | Eupatoriochromene Basic information |
| Product Name: | Eupatoriochromene | | Synonyms: | Eupatoriochromene;1-(2,2-Dimethyl-7-hydroxy-α-chromene-6-yl)ethanone;6-Acetyl-7-hydroxy-2,2-dimethyl-2H-1-benzopyran;Demethylencecalin;Des-O-methylencecalin;Methyl(2,2-dimethyl-7-hydroxy-2H-1-benzopyran-6-yl) ketone;Ethanone,1-(7-hydroxy-2,2-dimethyl-2H-1- benzopyran-6-yl)-;1-(7-hydroxy-2,2-dimethylchromen-6-yl)ethanone | | CAS: | 19013-03-7 | | MF: | C13H14O3 | | MW: | 218.25 | | EINECS: | | | Product Categories: | | | Mol File: | 19013-03-7.mol | 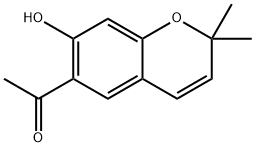 |
| | Eupatoriochromene Chemical Properties |
| Melting point | 80 °C | | Boiling point | 366.7±42.0 °C(Predicted) | | density | 1.154±0.06 g/cm3(Predicted) | | form | Off-white to yellowish solid. | | pka | 9.92±0.40(Predicted) | | LogP | 4.150 (est) |
| | Eupatoriochromene Usage And Synthesis |
| Uses | Eupatoriochromene is a chromene and has inhibitory activity against xanthine oxidase (XO)[1][2]. | | Definition | ChEBI: Eupatoriochromene is a 1-benzopyran. | | References | [1] G B Merrill?. Eupatoriochromene and encecalin, plant growth regulators from yellow starthistle (Centaurea solstitialis L.). J Chem Ecol.?1989 Jul;15(7):2073-87. DOI:10.3389/fnut.2021.737157
[2] Xin-Sheng Liu, et al. Chemical Compounds, Antioxidant Activities, and Inhibitory Activities Against Xanthine Oxidase of the Essential Oils From the Three Varieties of Sunflower ( Helianthus annuus L.) Receptacles. Front Nutr. 2021 Nov 19;8:737157. DOI:10.3389/fnut.2021.737157 |
| | Eupatoriochromene Preparation Products And Raw materials |
|