|
|
| | METHYL TETRAHYDRO-4-OXO-2H-THIOPYRAN-3-CARBOXYLATE Basic information |
| Product Name: | METHYL TETRAHYDRO-4-OXO-2H-THIOPYRAN-3-CARBOXYLATE | | Synonyms: | METHYL TETRAHYDRO-4-OXO-2H-THIOPYRAN-3-CARBOXYLATE;4-OXO-TETRAHYDRO-THIOPYRAN-3-CARBOXYLIC ACID METHYL ESTER;Methyl tetrahydro-4-oxo-2H-thiopyran-3-carboxylate ,96%;Methyl 4-oxotetrahydrothiopyran-3-carboxylate;3-(Carbomethoxy)tetrahydrothiopyran-4-one;3-Carbomethoxytetrahydro-1,4-thiapyrone;3-Methoxycarbonyl-4-thianone;methyl 4-oxo-tetrahydro-2H-thiopyran-3-carboxylate | | CAS: | 4160-61-6 | | MF: | C7H10O3S | | MW: | 174.22 | | EINECS: | 145-852-9 | | Product Categories: | | | Mol File: | 4160-61-6.mol | 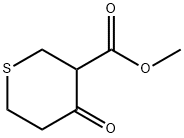 |
| | METHYL TETRAHYDRO-4-OXO-2H-THIOPYRAN-3-CARBOXYLATE Chemical Properties |
| Boiling point | 285℃ | | density | 1.244 | | Fp | 137℃ | | storage temp. | Sealed in dry,Room Temperature | | pka | 10.41±0.20(Predicted) | | form | Liquid or Solid | | color | Clear colorless to pale yellow |
| | METHYL TETRAHYDRO-4-OXO-2H-THIOPYRAN-3-CARBOXYLATE Usage And Synthesis |
| Chemical Properties | Light pink liquid | | Synthesis | General procedure for the synthesis of methyl tetrahydro-4-oxo-2H-thiopyran-3-carboxylate from dimethyl 3,3'-thiodipropionate: 2.7098 g of NaH (60%) was added to a dry 250 ml three-necked flask, followed by the addition of 40 ml of anhydrous tetrahydrofuran (THF) and stirring for 10 min at room temperature. Dimethyl 3,3'-thiodipropionate (10.1015 g) dissolved in THF was added slowly dropwise. After dropwise addition, the reaction mixture was heated to reflux for about 1 hour. The reflux was continued for 1 hour after rinsing the dropping funnel with 10 ml of THF. The reaction was stopped and cooled to room temperature. The pH of the reaction solution was adjusted to 6-7 with 2% hydrochloric acid and then extracted with dichloromethane (30 ml x 3). The organic layers were combined, washed with saturated sodium chloride solution, collected and dried over anhydrous sodium sulfate. After filtration, the solvent was removed under reduced pressure to give 7.5639 g of a yellow oily liquid product in 88.7% yield. | | References | [1] Synthesis, 2007, # 10, p. 1584 - 1586
[2] Patent: CN104086562, 2016, B. Location in patent: Paragraph 0136; 0137
[3] Patent: CN105153190, 2017, B. Location in patent: Paragraph 0134; 0165; 0166
[4] Organic Letters, 2018, vol. 20, # 19, p. 6104 - 6107
[5] Journal of the American Chemical Society, 1997, vol. 119, # 18, p. 4285 - 4291 |
| | METHYL TETRAHYDRO-4-OXO-2H-THIOPYRAN-3-CARBOXYLATE Preparation Products And Raw materials |
|