|
|
| | 7-AMINO-1,8-NAPHTHYRIDIN-2(8H)-ONE Basic information |
| Product Name: | 7-AMINO-1,8-NAPHTHYRIDIN-2(8H)-ONE | | Synonyms: | 2-AMino-1,8-naphthyridin-7-ol;7-AMino-1,8-naphthyridin-2-one;7-AMINO-1,8-NAPHTHYRIDIN-2(8H)-ONE;7-AMINO-1,8-NAPHTHYRIDIN-2-OL;7-amino-1,8-naphthyridin-2(1H)-one;2-AMINO-7-HYDROXY-1,8-NAPHTHYRIDINE;7-Amino-1,2-dihydro-1,8-naphthyridine-2-one;7-amino-1,8-naphthyridin-2(1H)-one(SALTDATA: FREE) | | CAS: | 1931-44-8 | | MF: | C8H7N3O | | MW: | 161.16 | | EINECS: | 217-688-5 | | Product Categories: | Amines;Aromatics;Heterocycles | | Mol File: | 1931-44-8.mol | 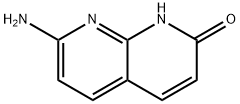 |
| | 7-AMINO-1,8-NAPHTHYRIDIN-2(8H)-ONE Chemical Properties |
| Melting point | >335°C (dec.) | | Boiling point | 415.6±38.0 °C(Predicted) | | density | 1.49±0.1 g/cm3(Predicted) | | storage temp. | Keep in dark place,Inert atmosphere,Room temperature | | solubility | Methanol (Slightly, Heated) | | form | Solid | | pka | 3.39±0.20(Predicted) | | color | Dark Yellow to Yellow-Brown | | InChI | InChI=1S/C8H7N3O/c9-6-3-1-5-2-4-7(12)11-8(5)10-6/h1-4H,(H3,9,10,11,12) | | InChIKey | NSPQTGOJGZXAJM-UHFFFAOYSA-N | | SMILES | N1C2C(=CC=C(N)N=2)C=CC1=O |
| Hazard Codes | Xn | | Risk Statements | 22 |
| | 7-AMINO-1,8-NAPHTHYRIDIN-2(8H)-ONE Usage And Synthesis |
| Chemical Properties | Yellow-Brown Solid | | Synthesis | 2,6-diaminopyridine (20 g, 0.18 mol) and DL-malic acid (27 g, 0.2 mol) were used as raw materials in a 500 mL three-necked round-bottomed flask equipped with a dosing funnel, thermometer and mechanical stirrer. The mixture was cooled in an ice-water bath, and concentrated sulfuric acid (100 mL) was added slowly dropwise, with the rate of dropwise acceleration controlled to keep the reaction temperature from exceeding 45 °C. Subsequently, the addition funnel was replaced with a reflux condenser and the reaction mixture was heated to 110°C and maintained for 3 hours. Upon completion of the reaction, the solution was transferred to a 1 L beaker, cooled to 0 °C via an ice-water bath, and adjusted to pH=8 by slowly adding aqueous NH4OH (300 mL).The crude product was collected by vacuum filtration and the filter cake was washed with 2 L of water. The solid product was ground with a 1:9 (v:v) water:methanol mixture and collected again by vacuum filtration to afford 7-amino-2-hydroxy-1,8-diazanaphthalene as a tan powder in 25 g (86% yield). The product was structurally confirmed by 1H NMR (500 MHz, DMSO-d6) and 13C NMR (126 MHz, DMSO-d6) as well as mass spectrometry (ESI, CH3OH). | | References | [1] Journal of Organic Chemistry, 1981, vol. 46, # 5, p. 833 - 839
[2] Organic and Biomolecular Chemistry, 2012, vol. 10, # 13, p. 2578 - 2589
[3] Organic and Biomolecular Chemistry, 2012, vol. 10, # 25, p. 4899 - 4906
[4] Organic and Biomolecular Chemistry, 2012, vol. 10, # 1, p. 90 - 95
[5] Organic and Biomolecular Chemistry, 2014, vol. 12, # 33, p. 6500 - 6506 |
| | 7-AMINO-1,8-NAPHTHYRIDIN-2(8H)-ONE Preparation Products And Raw materials |
|