|
|
| | 2-(6-broMopyridin-2-yl)propan-2-ol Basic information |
| Product Name: | 2-(6-broMopyridin-2-yl)propan-2-ol | | Synonyms: | 2-(6-broMopyridin-2-yl)propan-2-ol;2-(6-Bromo-2-pyridyl)-2-propanol;2-Pyridinemethanol, 6-bromo-α,α-dimethyl-;2-(6-broMopyridin-2-yl)propan-2-ol ISO 9001:2015 REACH;2-(6-bromopyridine) -2-propanol | | CAS: | 638218-78-7 | | MF: | C8H10BrNO | | MW: | 216.08 | | EINECS: | | | Product Categories: | | | Mol File: | 638218-78-7.mol | 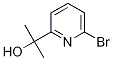 |
| | 2-(6-broMopyridin-2-yl)propan-2-ol Chemical Properties |
| Boiling point | 273.2±25.0 °C(Predicted) | | density | 1.474±0.06 g/cm3(Predicted) | | storage temp. | under inert gas (nitrogen or Argon) at 2-8°C | | pka | 13.23±0.29(Predicted) | | Appearance | Colorless to off-white Solid-liquid mixture | | InChI | InChI=1S/C8H10BrNO/c1-8(2,11)6-4-3-5-7(9)10-6/h3-5,11H,1-2H3 | | InChIKey | OXSDDDKLMCHNHF-UHFFFAOYSA-N | | SMILES | CC(C1=NC(Br)=CC=C1)(O)C |
| | 2-(6-broMopyridin-2-yl)propan-2-ol Usage And Synthesis |
| Synthesis | GENERAL STEPS: In a dry 250 mL round-bottomed flask equipped with a stir bar, septum and temperature probe, 1.6 M hexane solution of n-butyllithium (31.2 mL, 50 mmol) was added. The reaction system was cooled to -76 °C in a dry ice-acetone bath. Subsequently, THF (30 mL) was added to the reaction vial and a solution of 2,6-dibromopyridine (11.5 g, 50 mmol) in THF (60 mL) was added slowly dropwise by syringe, controlling the reaction temperature below -60 °C. Stirring of the dark yellow-brown solution was continued in a dry ice bath for 30 min, and then acetone (6 mL, 80 mmol) was added. The reaction mixture was stirred in a dry ice bath for 15 minutes and then slowly warmed to room temperature. After 1 hour of reaction, the reaction was quenched by careful addition of 5% aqueous ammonium chloride solution (50 mL). The reaction mixture was extracted with dichloromethane, the organic phases were combined and the solvent evaporated to give 2-(6-bromopyridin-2-yl)propan-2-ol (10.6 g, 98% yield) as an orange oil. The mass spectrum (MS) showed m/z: 216 and 218 (M+H)+. | | References | [1] Patent: WO2009/131814, 2009, A2. Location in patent: Page/Page column 40
[2] Patent: US2013/178478, 2013, A1. Location in patent: Paragraph 0461; 0462
[3] Patent: WO2014/60371, 2014, A1. Location in patent: Page/Page column 76
[4] Chemical Communications, 2016, vol. 52, # 74, p. 11056 - 11059
[5] Patent: WO2004/814, 2003, A1. Location in patent: Page 50 |
| | 2-(6-broMopyridin-2-yl)propan-2-ol Preparation Products And Raw materials |
|