ChemicalBook >?? ???? >(2-?????)??????
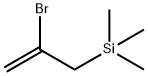
(2-?????)??????
|
|
(2-?????)?????? ??
- ?? ?
- 82-85 °C/60 mmHg (lit.)
- ??
- 1.121 g/mL at 25 °C (lit.)
- ???
- n
20/D 1.462(lit.)
- ???
- 87 °F
- ?? ??
- 2-8°C
- ???
- ???, ???, ???, ?????????, ??? ???? ?? ??????.
- ??? ??
- liquid
- BRN
- 3600686
??
- ?? ? ?? ??
- ?? ? ???? ?? (GHS)
| ??? ?? | Xi | ||
|---|---|---|---|
| ?? ???? ?? | 10-36/37/38 | ||
| ????? | 16-26-36 | ||
| ????(UN No.) | UN 1993 3/PG 3 | ||
| WGK ?? | 3 | ||
| F ?????? | 8-10 | ||
| HS ?? | 2931.90.9010 | ||
| ?? ?? | 3.2 | ||
| ???? | III |
(2-?????)?????? C??? ??, ??, ??
??? ??
bp 46–50°C/20 mmHg,64–65°C/38– 39 mmHg.??
2-Bromo-3-trimethylsilyl-1-propene can be used as synthon for CH2=C?CH2TMS1–3 and CH2=CBrC?H2;2 for synthesis of 1-trimethylsilylmethyl-substituted 1,3-butadienes.The 1-trimethylsilylmethylvinyl anion CH2=C(M)CH2TMS (2) (M = Li, Mg, Cu, etc.), readily prepared from 2-bromo-3-trimethylsilyl-1-propene (1) under typical conditions, allows the introduction of the synthetically useful 1-trimethylsilylmethylvinyl group to a wide variety of substrates. Ring opening of 1-butene oxide with the Grignard reagent (2) (M = MgBr) in the presence of copper(I) iodide gives only one regioisomer. Subsequent desilylative oxidation of this allyl alcohol to α-methylene-γ-lactones provides further utility of (1) as a 1-hydroxymethylvinyl anion equivalent, i.e. CH2=?C?CH2OH (eq 1).Alternatively, the alcohol from trans-2,3-epoxybutane provides a route to the unstable sixmembered β,γ-unsaturated lactone (eq 2).The copper-catalyzed 1,4-addition to the typically unreactive mesityl oxide proceeds smoothly. The versatility of the allylsilane moiety is again illustrated in the ethylaluminum dichloride-induced cyclization of the adduct to a tertiary cyclopentanol in high yield (eq 3).

?? ??
reaction of 2,3-dibromopropene with lithium (trimethylsilyl)cuprate in HMPA at 0°C (63–90%);(2) reaction of 2,3-dibromopropene with trichlorosilane in the presence of trichlorosilane and copper( I) chloride, followed by treatment with methylmagnesium bromide (63–71%).Purification Methods
It is fractionally distilled through an efficient column. It is flammable. [Trost & Chan J Am Chem Soc 104 3733 1982, Trost & Coppola J Am Chem Soc 104 6879 1982.](2-?????)?????? ?? ?? ? ???
???
?? ??
(2-?????)?????? ?? ??
???( 65)?? ??
| ??? | ?? | ??? | ?? | ?? ? | ?? |
|---|---|---|---|---|---|
| career henan chemical co | +86-0371-86658258 +8613203830695 |
factory@coreychem.com | China | 29804 | 58 |
| Henan Alfa Chemical Co., Ltd | +8615838112936 |
alfa10@alfachem.cn | China | 12959 | 58 |
| Alfa Chemistry | +1-5166625404; |
Info@alfa-chemistry.com | United States | 20405 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418684 +8618949823763 |
sales@tnjchem.com | China | 25356 | 58 |
| LEAP CHEM CO., LTD. | +86-852-30606658 19957148339; |
market18@leapchem.com | China | 24727 | 58 |
| Hebei Chuanghai Biotechnology Co., Ltd | +8615531151365 |
mina@chuanghaibio.com | China | 18126 | 58 |
| ShenZhen Trendseen Biological Technology Co.,Ltd. | 13417589054 |
trendseenbio@gmail.com | China | 11681 | 58 |
| LEAPCHEM CO., LTD. | +86-852-30606658 |
market18@leapchem.com | China | 43340 | 58 |
| Aladdin Scientific | |
tp@aladdinsci.com | United States | 57505 | 58 |
| Chemwill Asia Co.,Ltd. | 86-21-51086038 |
chemwill_asia@126.com | CHINA | 23912 | 58 |