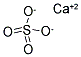ChemicalBook >?? ???? >????(??)
????(??)
|
|
????(??) ??
- ???
- 1450 °C
- ??
- 2.32
- ??? ??
- ???? ?? ?? ???
- ??
- ???
- Dielectric constant
- 1.5(Ambient)
??
| ??? ?? | T,N | ||
|---|---|---|---|
| ?? ???? ?? | 49-42/43-51/53-68-50/53-60 | ||
| ????? | 53-22-36/37-45-60-61 | ||
| ????(UN No.) | UN 3077 9/PG 3 | ||
| OEB | B | ||
| OEL | TWA: 10 mg/m3 (total) | ||
| WGK ?? | 1 | ||
| RTECS ?? | WS6920000 | ||
| F ?????? | 3 | ||
| ?? | human,TCLo,inhalation,194gm/m3/10Y- (194000mg/m3),SENSE ORGANS AND SPECIAL SENSES: OTHER CHANGES: OLFACTIONLUNGS, THORAX, OR RESPIRATION: OTHER CHANGESLUNGS, THORAX, OR RESPIRATION: FIBROSING ALVEOLITIS,Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 11(10), Pg. 23, 1967. |
????(??) C??? ??, ??, ??
??
????, ?????, ??? ??? ?????? ? ??? ??? ?? ???? ???? ??? ???? ?? ??? ????? ??? ????? ?? ???? ?????.??
Phosphogypsum is a moist, fine powder with a free water content of ca. 20 – 30 % and considerable amounts of impurities, the exact impurities and their amounts depending on the rock and the specific process. The radioactive substances present in small amounts in sedimentary phosphate rock are partly transferred to the phosphogypsum as 226Ra, leading to slightly increased radioactivity of such gypsums. About 1.7 t of gypsum is obtained per tonne of raw phosphate, corresponding to 5 t of gypsum per tonne of phosphorus pentoxide produced.??
Anhydrous: insoluble anhydrite is used in cement formulations and as a paper filler. Soluble anhydride, because of its strong tendency to absorb moisture, is useful as a drying agent for solids, organic liquids and gases; the desiccant used in laboratory and industry is known under the name Drierite. This material can be regenerated repeatedly and reused without noticeable decrease in its desiccating efficiency. The hemihydrate is used for wall plasters; wallboard; tiles and blocks for the building industry; moldings; statuary; in the paper industry. The dihydrate is used in the manufacture of portland cement; in soil treatment to neutralize alkali carbonates and to prevent loss of volatile and dissolved nitrogenous compounds by volatilization and leaching; for the manufacture of plaster of Paris, artificial marble; as a white pigment, filler or glaze in paints, enamels, pharmaceuticals, paper, insecticide dusts, yeast manufacture, water treatment, polishing powders; in the manufacture of sulfuric acid, CaC2, (NH4)2SO4, porous polymers. Pharmaceutic aid (in plaster casts).??
A powdered mixture of calcium silicates and aluminates, which is made by heating limestone (CaCO3) with clay, and grinding the result. When mixed with water, reactions occur with the water (hence the name hydraulic cement) and a hard solid aluminosilicate is formed.?? ??
White or nearly white, odorless, crystalline solid.?? ???
CALCIUM SULFATE HEMIHYDRATE is generally of low reactivity. Can serve as an oxidizing agent under forcing conditions. Incompatible with aluminum (at high temperatures) and diazomethane.???
Calcium sulphate (CaSO4) is a white solid that occurs naturally as a mineral anhydrite. It is found more commonly as dihydrate, called gypsum (CaSO4·2H2O). When heated, gypsum loses water at 128°C to give a hemihydrate (2CaSO4·H2O), also known as plaster of Paris.Calcium sulphate is sparingly soluble in water and makes water permanently hard. CaSO4 is used in the manufacture of certain paints, ceramics and paper. Its naturally occurring form is used in sulphuric acid manufacture.
Gypsum is the cheapest and the most useful material in reclamation of sodic soils.Calcium, solubilized from gypsum, replaces sodium, leaving behind the watersoluble sodium sulphate, which is leached out as a result of the following reactions in the soil:
Since both reactions are reversible, adequate leaching arrangements have to be made to remove sodium sulphate.
The application of about 40 t/ha of gypsum in Nevada (USA) was seen to increase water infiltration and the depth of water penetration substantially. These two measures increased hay yield up to 2.3 t/ha per year.
Gypsum requirement (GR) is the amount of gypsum necessary to be added to reclaim soil and is calculated using the formula:
The gypsum requirement is equivalent to (Nax)×4.50 metric tons of gypsum per hectare for a 30 cm fixed depth, where Na, is the milliequivalent of exchangeable sodium to be replaced by calcium from the added gypsum.
????(??) ?? ?? ? ???
???
?? ??
????(??) ?? ??
???( 94)?? ??
| ??? | ?? | ??? | ?? | ?? ? | ?? |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21622 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
factory@coreychem.com | China | 29804 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 |
sales@tnjchem.com | China | 34563 | 58 |
| Hebei Fengjia New Material Co., Ltd | +86-0311-87836622 +86-18712993135 |
sales01@tairunfaz.com | China | 8051 | 58 |
| XIAMEN AMITY INDUSTRY AND TRADE CO., LTD. | +8618950047208 |
ellena@amitychem.com | China | 43416 | 58 |
| GetChem Co., Ltd. | +8619153197336 |
info@getchem.com | China | 7878 | 58 |
| Mainchem Co., Ltd. | -- |
sarah@mainchem.com | China | 6547 | 58 |
| Chemwill Asia Co.,Ltd. | 86-21-51086038 |
chemwill_asia@126.com | CHINA | 23912 | 58 |
| Chizhou Kailong Import and Export Trade Co., Ltd. | |
xg01_gj@163.com | China | 9484 | 50 |
| Shaanxi Dideu Medichem Co. Ltd | 029-029-81024372 17792795018 |
1027@dideu.com | China | 10010 | 58 |