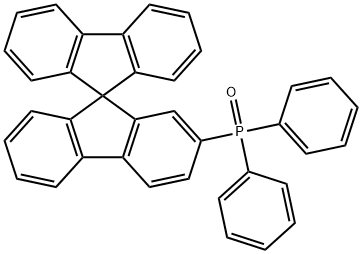
SPPO1,9,9-????????-2-?-???-???????
SPPO1,9,9-????????-2-?-???-??????? ??? ???
?? ??:
1125547-88-7
???:
SPPO1,9,9-????????-2-?-???-???????
???(??):
SPPO1,9,9-????????-2-?-???-???????
???:
SPPO1 , 9,9-spirobifluoren-2-yl-diphenyl-phosphine oxide
???(??):
SPPO1;9,9-spirobifluoren-2-yl-diphenyl-phosphine oxide;9,9’-spiro[fluoren]-2-yl dipenyl phosphine oxide;9,9'-Spirobi[fluoren]-2-yldiphenylphosphine oxide;9,9'-Spirobi[fluoren]-7-yldiphenylphosphine oxide;Diphenyl-9,9'-spirobi[9H-fluoren]-2-ylphosphine oxide;SPPO1 , 9,9-spirobifluoren-2-yl-diphenyl-phosphine oxide;Phosphine oxide, diphenyl-9,9'-spirobi[9H-fluoren]-2-yl-;Diphenyl[9,9'-spirobi[9H-fluoren]-2-yl]phosphine Oxide (This product is only available in Japan.)
CBNumber:
CB32548185
???:
C37H25OP
??? ??:
516.57
MOL ??:
1125547-88-7.mol
SPPO1,9,9-????????-2-?-???-??????? ??
???
236℃
?? ?
719.6±60.0 °C(Predicted)
??
1.32±0.1 g/cm3(Predicted)
??? ??
powder/crystals
??
???
Photoluminescence
λem 347 nm (dichloromethane)
Absorption
λmax 317 nm (dichloromethane)
??
SPPO1,9,9-????????-2-?-???-??????? C??? ??, ??, ??
??
SPPO1, 9,9-Spirobifluoren-2-yl-diphenyl-phosphine oxide has a spirobifluorene structure with an electron deficient polar diphenyl-phosphine oxide moiety attached. The polarity in the P=O double bond can lead to a high triplet energy state, which is highly desirable in host materials for an efficient host-to guest energy transfer in an OLED.
SPPO1,9,9-????????-2-?-???-??????? ?? ?? ? ???
???
?? ??
SPPO1,9,9-????????-2-?-???-??????? ?? ??
???( 23)?? ??
China 19
Japan 1
United Kingdom 1
United States 2
Global 23