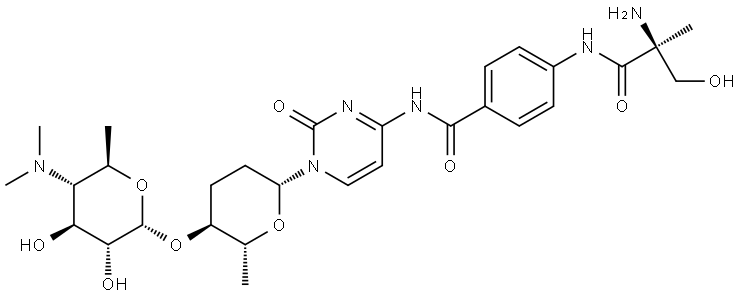
Amicetin-A
- CAS No.
- 17650-86-1
- Chemical Name:
- Amicetin-A
- Synonyms
- amicetin;Amicetin-A;Amomycin A;Benzamide, 4-[[(2S)-2-amino-3-hydroxy-2-methyl-1-oxopropyl]amino]-N-[1-[(2R,5S,6R)-5-[[4,6-dideoxy-4-(dimethylamino)-α-D-glucopyranosyl]oxy]tetrahydro-6-methyl-2H-pyran-2-yl]-1,2-dihydro-2-oxo-4-pyrimidinyl]-
- CBNumber:
- CB61244964
- Molecular Formula:
- C29H42N6O9
- Molecular Weight:
- 618.68
- MDL Number:
- MFCD01674585
- MOL File:
- 17650-86-1.mol
- MSDS File:
- SDS
| Melting point | 244-245° |
|---|---|
| alpha | D24 +116.5° (c = 0.5 in 0.1N HCl) |
| Boiling point | 660.6°C (rough estimate) |
| Density | 1.2721 (rough estimate) |
| refractive index | 1.6400 (estimate) |
| pka | pKa = 10.4, 7.0(at 25℃) |
| EWG's Food Scores | 1 |
| FDA UNII | 0909X15C85 |
SAFETY
Risk and Safety Statements
| Toxicity | LD50 of citrate complex, pH 6 (mg/kg) in mice: ~90 i.v., 600-700 s.c.; in rats: ~200 i.v., ~600 s.c. (DeBoer) |
|---|
Amicetin-A Chemical Properties,Uses,Production
Uses
Amicetin is a potent antibiotic. Amicetin shows antibiotic activities against gram-positive bacteria. Amicetin inhibits protein synthesis[1][2][3].
Enzyme inhibitor
These pyrimidine-containing glycoside antibiotic (FWAmicetin-A = 617.69 g/mol (free base); CAS 17650-86-1; FWAmicetin-B = 532.59 g/mol (free base)) from Streptomyces vinaceus-drappus and S. fasciculatus inhibits protein biosynthesis, exerting its bacteriostatic most potently on Grampositive bacteria. Amicetin B, also called plicacetin, was isolated from Streptomyces plicatus and has the identical structure minus the amethylseryl residue. It is weaker in action compared to amicetin A. Target(s): peptidyltransferase; peptidyl-tRNA hydrolase, or aminoacyl-tRNA hydrolase; ribonuclease P; protein biosynthesis.
References
[1] HINUMA Y, et al. An amicetin (sacromycin) from Streptomyces sp. No. 5223. Studies on Streptomyces antibiotics. XXXVI. J Antibiot (Tokyo). 1955 Nov;8(5):148-52. PMID:13306673
[2] Fu, J., et al. Total Synthesis of Nucleoside Antibiotics Amicetin, Plicacetin, and Cytosaminomycin A—D. Chinese Journal of Chemistry, 2021, 39(10), 2679-2684.
[3] Ennis, H. L. et, al. Polysome Metabolism in Escherichia coli: Amicetin, an Antibiotic That Stabilizes Polysomes. Antimicrobial Agents and Chemotherapy, 1972, 1(3), 204-209. doi:10.1128/aac.1.3.204. DOI:10.1128/AAC.1.3.204
Amicetin-A Preparation Products And Raw materials
Raw materials
Preparation Products
Amicetin-A Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| BOC Sciences | +1-631-485-4226 | inquiry@bocsci.com | United States | 19552 | 58 |
| Shaanxi Cuikang Pharmaceutical Technology Co., Ltd | +8618791163155 | 18791163155@163.com | China | 3439 | 58 |
| Zhejiang Huida Biotech Co., LTD | 0571-89903882 18679456698 | mingxinzhu@huidabiotech.com | China | 3341 | 58 |
| Zhejiang Huida Biotech Co., LTD | 0571-0571-89903882 15990081639 | sunshixuan@huidabiotech.com | China | 3705 | 58 |
| Biosynth Biological Technology (Suzhou) Co Ltd | 51288865780 | sales@biosynth.com | China | 6051 | 58 |