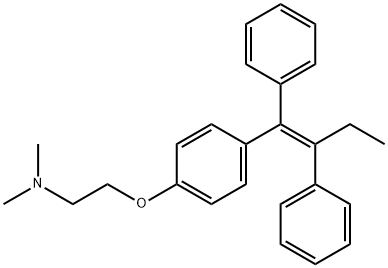
Tamoxifen
|
|
Tamoxifen Eigenschaften
- Schmelzpunkt:
- 97-98 °C(lit.)
- Siedepunkt:
- 501.18°C (rough estimate)
- Dichte
- 1.0630 (rough estimate)
- Dampfdruck
- 0Pa at 25℃
- Brechungsindex
- 1.6000 (estimate)
- storage temp.
- 2-8°C
- L?slichkeit
- H2O: insoluble <0.1% at 20°C
- pka
- pKa 8.71(H2O t = 25 I = 0.025) (Uncertain)
- Aggregatzustand
- Solid
- Farbe
- Crystals from pet ether
- Wasserl?slichkeit
- Insoluble in water. Soluble in methanol, ethanol, propanol or propylene glycol.Soluble in dimethyl sulfoxide, dichloromethane and ethanol. Insoluble in water.
- Merck
- 13,9137
- Stabilit?t:
- Light Sensitive
- InChIKey
- NKANXQFJJICGDU-QPLCGJKRSA-N
- LogP
- 6.3 at 20℃
- CAS Datenbank
- 10540-29-1(CAS DataBase Reference)
- IARC
- 1 (Vol. 66, 100A) 2012
- EPA chemische Informationen
- Tamoxifen (10540-29-1)
Sicherheit
- Risiko- und Sicherheitserkl?rung
- Gefahreninformationscode (GHS)
| Kennzeichnung gef?hrlicher | T,Xi | ||
|---|---|---|---|
| R-S?tze: | 45-60-61-64-36/37/38 | ||
| S-S?tze: | 53-45-36-26 | ||
| WGK Germany | 3 | ||
| RTECS-Nr. | KR5919600 | ||
| HS Code | 29221990 | ||
| Giftige Stoffe Daten | 10540-29-1(Hazardous Substances Data) | ||
| Toxizit?t | LD50 orl-rat: 4100 mg/kg DRFUD4 9,186,84 |
| Bildanzeige (GHS) |
 
|
||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alarmwort | Achtung | ||||||||||||||||||||||||||||
| Gefahrenhinweise |
|
||||||||||||||||||||||||||||
| Sicherheit |
|
Tamoxifen Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R45:Kann Krebs erzeugen.R60:Kann die Fortpflanzungsf?higkeit beeintr?chtigen.
R61:Kann das Kind im Mutterleib sch?digen.
R64:Kann S?uglinge über die Muttermilch sch?digen.
R36/37/38:Reizt die Augen, die Atmungsorgane und die Haut.
S-S?tze Betriebsanweisung:
S53:Exposition vermeiden - vor Gebrauch besondere Anweisungen einholen.S45:Bei Unfall oder Unwohlsein sofort Arzt zuziehen (wenn m?glich, dieses Etikett vorzeigen).
S36:DE: Bei der Arbeit geeignete Schutzkleidung tragen.
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.
Beschreibung
In 1966, ICI Pharmaceuticals (now AstraZeneca) first synthesized tamoxifen in the hope of developing a morning-after contraceptive pill. The UK patent for this compound was in place in 1962, whereas the US patent was repeatedly denied until the 1980s. Tamoxifen was approved for a fertility treatment but it was not proven as useful in regulating human contraception. Even though there was a link between estrogen and breast cancer, developing a cancer treatment was not a priority at the time. In 1971, the first clinical study showed a convincing effect of tamoxifen in treating advanced breast cancer. From 1971 to 1977, this drug was neither clinically nor financially remarkable. In 1980s, however, publications first showed that tamoxifen, in addition to chemotherapy, improved survival for patients with early stage breast cancer. In 1998, the meta-analysis by the Oxford-based Early Breast Cancer Trialists’ Collaborative Group showed that tamoxifen did indeed save lives in early breast cancer. In 2001, tamoxifen sales were over $1.024 billion. Since the expiration of the patent in 2002, it is now widely available as a generic drug. By 2004, tamoxifen was the best selling hormonal drug for the treatment of breast cancer.Chemische Eigenschaften
White Crystalline SolidVerwenden
A nonsteroidal estrogen antagonist of interest in the treatment of some forms of breast cancer. Tamoxifen is a Protein Kinase C inhibitor, and induces apoptosis in human malignant glioma cell linesIndications
Tamoxifen (Nolvadex) is a synthetic antiestrogen used in the treatment of breast cancer. Normally, estrogens act by binding to a cytoplasmic protein receptor, and the resulting hormone–receptor complex is then translocated into the nucleus, where it induces the synthesis of ribosomal RNA (rRNA) and messenger RNA (mRNA) at specific sites on the DNA of the target cell. Tamoxifen also avidly binds to estrogen receptors and competes with endogenous estrogens for these critical sites. The drug–receptor complex has little or no estrogen agonist activity.Tamoxifen directly inhibits growth of human breast cancer cells that contain estrogen receptors but has little effect on cells without such receptors.Weltgesundheitsorganisation (WHO)
Tamoxifen is an anti-estrogen agent used mainly to treat breast cancer. Tamoxifen is listed in the WHO Model List of Essential Drugs.Allgemeine Beschreibung
Tamoxifen is a selective estrogen response modifier (SERM), protein kinase C inhibitor and anti-angiogenetic factor. Tamoxifen is a prodrug that is metabolized to active metabolites 4-hydroxytamoxifen (4-OHT) and endoxifen by cytochrome P450 isoforms CYP2D6 and CYP3A4. In breast cancer, the gene repressor activity of tamoxifen against ERBB2 is dependent upon PAX2. Blocks estradiol-stimulated VEGF production in breast tumor cells.Mechanism of action
Tamoxifen is slowly absorbed, and maximum serum levels are achieved 4 to 7 hours after oral administration. The drug is concentrated in estrogen target tissues, such as the ovaries, uterus, vaginal epithelium, and breasts. Hydroxylation and glucuronidation of the aromatic rings are the major pathways of metabolism; excretion occurs primarily in the feces.Pharmakokinetik
Circulating levels of the demethylated metabolite at steady state are up to twice the level of the parent drug, because the elimination half-life of N-demethyl tamoxifen is 14 days, compared with 7 days for tamoxifen. Tamoxifen demonstrates only weak estrogenic effects at several sites, including the endometrium and bone, and on the lipid profile. Tamoxifen undergoes rapid N-dem ethylation to its major metabolite, N-dem ethyltamoxifen, by CYP3A4 and via CYP2D6 to its minor metabolite, 4-hydroxytam oxifen. Evidence suggests that 4-hydroxytamoxifen is the active metabolite of tamoxifen, with a higher binding affinity than the parent drug for the ERClinical Use
Tamoxifen is a SERM that is used as an antiestrogen in the treatment of estrogen-dependent breas Tcancer following prim ary treatment (c hemotherapy and/or surgery).Nebenwirkungen
Tamoxifen administration is associated with few toxic side effects, most frequently hot flashes (in 10–20% of patients) and occasionally vaginal dryness or discharge. Mild nausea, exacerbation of bone pain, and hypercalcemia may occur.Sicherheitsprofil
Confirmed human carcinogen. Moderately toxic by ingestion and intraperitoneal routes. Human systemic effects by an unspecified route: nausea or vomiting, leukopenia, thrombocytopenia, and skin changes. An experimental teratogen. Other experimental reproductive effects. Human mutation data reported. When heated to decomposition it emits toxic fumes of NOx.Carcinogenicity
Tamoxifen is known to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in humans.Tamoxifen Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
Tamoxifen Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 443)Lieferanten
| Firmenname | Telefon | Land | Produktkatalog | Edge Rate | |
|---|---|---|---|---|---|
| Wuhan JiyunZen Tech Co., Ltd. | +86-18062099985 |
Amyjiyunzen@yeah.net | China | 663 | 58 |
| Hebei Bonster Technology Co.,Limited | +86-13315996897 |
bsterltd.wendy@gmail.com | China | 792 | 58 |
| Shandong Huisheng Import & Export Co., Ltd. | +86-13176845580 |
da@zhongda-biotech.com | China | 232 | 58 |
| Hubei KDG Web Science&Technology Co., Ltd | 18672314556 +8618672314556 |
sales@kdg-tech.com | China | 933 | 58 |
| Wuhan Kejiefeng Trading Co.,Ltd | +undefined18827003015 |
dong19981022@gmail.com | China | 177 | 58 |
| Hebei Mujin Biotechnology Co.,Ltd | +86-13288715578 |
sales@hbmojin.com | China | 12648 | 58 |
| Wuhan Cell Pharmaceutical Co., Ltd | +86-13129979210 |
sales@cellwh.com | China | 376 | 58 |
| Anhui Zhongda Biotechnology Co., Ltd | +8619956560829 |
justine@zhongda-biotech.com | China | 286 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 |
daisy@anhuiruihan.com | China | 973 | 58 |
| Hebei Anlijie Biotechnology Co., Ltd | +8619031013551 |
ably@aljbio.com | China | 144 | 58 |
10540-29-1(Tamoxifen)Verwandte Suche:
Chlordiphenylphosphin
Dimethoxydiphenylsilan
Dimethoxydimethylsilan
Diphenylsilan
tert-Butylchlordiphenylsilan
Phenoxybenzamin
Diphenylphosphin
Diethoxydiphenylsilan
(2E,4E)-Undeca-2,4-dienal
Biphenyl
1,5-Diphenylcarbonohydrazid
TAMOXIFEN CITRATE(RG)
(Z)-(2-(4-(1,2-Diphenylbut-1-enyl)phenoxy)dimethylammonium-dihydrogen-2-hydroxypropan-1,2,3-tricarboxylat
Ethyldimethylamin
Tamoxifen
TRANSFAT
- (Z)-1-(p-Dimethylaminoethoxyphenyl)-1,2-diphenyl-1-butene, trans-2-[4-(1,2-Diphenyl-1-butenyl)phenoxy]-N,N-dimethylethylamine
- 2-[4-[(Z)-1,2-Di(phenyl)but-1-enyl]phenoxy]-N,N-dimethylethanamine
- 2-[4-[(Z)-1,2-Diphenyl-1-butenyl]phenoxy]-N,N-dimethylethanamine
- 2-[p-[(Z)-1,2-Diphenyl-1-butenyl]phenyloxy]-N,N-dimethylethanamine
- N,N-Dimethyl-2-[p-[(Z)-1,2-diphenyl-1-butenyl]phenoxy]ethanamine
- C07108
- Tamoxifen,(Z)-1-(p-Dimethylaminoethoxyphenyl)-1,2-diphenyl-1-butene, trans-2-[4-(1,2-Diphenyl-1-butenyl)phenoxy]-N,N-dimethylethylamine
- 1-p-β-DiMethylaMinoethoxyphenyl-trans-1,2-diphenylbut-1-ene
- (Z)-2-(4-(1,2-diphenylbut-1-en-1-yl)phenoxy)-N,N-diMethylethanaMine
- TaMoxiefen
- EthanaMine,2-[4-[(1Z)-1,2-diphenyl-1-buten-1-yl]phenoxy]-N,N-diMethyl-
- (z)-2-(4-(1,2-diphenyl-1-butenyl)phenoxy)phenoxy)-n,n-dimethylethanamine
- (z)-2-(para-(1,2-diphenyl-1-butenyl)phenoxy)-n,n-dimethylamine
- 1-para-beta-dimethylaminoethoxyphenyl-trans-1,2-diphenylbut-1-ene
- 1-p-beta-dimethylaminoethoxyphenyl-trans-1,2-diphenylbut-1-ene
- Tamoxifen, >=99%
- Tamoxifen(ICI46,474)
- (Z)-1-(4-Dimethylaminoethoxyphenyl)-1,2-diphenyl-1-butene
- TAMOXIFEN
- TRANS-2-[4-(1,2-DIPHENYL-1-BUTENYL)PHENOXY]-N,N-DIMETHYLETHYLAMINE
- (Z)-2-[4-(1,2-DIPHENYL-1-BUTENYL)PHENOXY]-N,N-DIMETHYLETHANAMINE
- [Z]-1-[P-DIMETHYLAMINOETHOXYPHENYL]-1,2-DIPHENYL-1-BUTENE
- Genox
- Tamoxen
- tamoxifen free base
- Ethanamine, 2-4-(1Z)-1,2-diphenyl-1-butenylphenoxy-N,N-dimethyl-
- ici47699
- n-dimethyl-2-(p-(1,2-diphenyl-1-butenyl)phenoxy)-(z)-ethylamin
- Nolvadex-D
- tamoxifen(z)
- tamoxifendrugstandardsolution
- trans-tamoxifen
- (Z)-2-(4-(1,2-diphenylbut-1-en-1-yl)phenoxy)-N,N-dimethylethan-1-amine
- TAMOXIFEN (ICI 47699)
- 2-[4-[(1Z)-1,2-Diphenyl-1-buten-1-yl]phenoxy]-N,N-dimethylethanamine
- Tamoxiphen CAS NO.10540-29-1
- Tamoxifen citrate for performance test CRS
- Tamoxifen citrate CRS
- Steroids Raw Powder Tamoxife
- Tamoxifen USP/EP/BP
- Tamoxifen (1.0 mg/mL in Methanol)
- Tamoxifen Nolvadex
- Mammaton
- Novaldex
- TAMOXIFEN BASE
- z-tamoxifen
- amoxifen
- 4’-Hydroxy Tamoxifen-d6 (contains up to 10% E isomer)
- (Z)-2-[4-(1,2-Diphenyl-1-butenyl)phenoxy]-N,N-dimethylethylamine
- Tamoxifen in methanol
- Tamoxifen solution
- Tamoxifen 20mg
- Tamoxifen - Bio-X ?
- IC|47699
- T5648-1G
- MeRCK 10540-29-1
- 10540-29-1
- C26H28NO