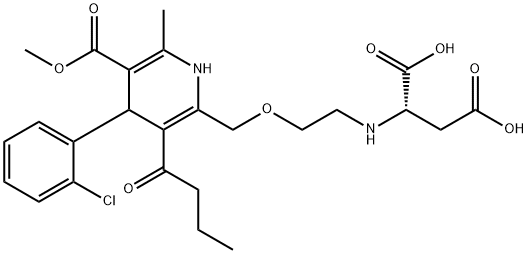
Amlodipine Impurity 79
|
|
Amlodipine Impurity 79 Eigenschaften
- Siedepunkt:
- 660.2±55.0 °C(Predicted)
- Dichte
- 1.294±0.06 g/cm3(Temp: 20 °C; Press: 760 Torr)(Predicted)
- pka
- 2.13±0.23(Predicted)
Sicherheit
Amlodipine Impurity 79 Chemische Eigenschaften,Einsatz,Produktion Methoden
Amlodipine Impurity 79 Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
Amlodipine Impurity 79 Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 2)Lieferanten
| Firmenname | Telefon | Land | Produktkatalog | Edge Rate | |
|---|---|---|---|---|---|
| China National Standard Pharmaceutical Corporation Limited | +8615391658522 |
overseasales@yongstandards.com | China | 11922 | 58 |
| Hubei De'ao Chemical Research Pharmaceutical Technology Co., Ltd | 18502726233; 18502726233 |
3641133981@qq.com | China | 9713 | 58 |
- Amlodipine Impurity 79
- N-[2-[[4-(2-Chlorophenyl)-1,4-dihydro-5-(methoxycarbonyl)-6-methyl-3-(1-oxobutyl)-2-pyridinyl]methoxy]ethyl]-L-aspartic acid
- 1220102-11-3