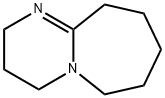
1,8-Diazabicyclo(5.4.0)undec-7-en
|
|
1,8-Diazabicyclo(5.4.0)undec-7-en Eigenschaften
- Schmelzpunkt:
- -70 °C
- Siedepunkt:
- 80-83 °C0.6 mm Hg(lit.)
- Dichte
- 1.019 g/mL at 20 °C(lit.)
- Dampfdruck
- 5.3 mm Hg ( 37.7 °C)
- Brechungsindex
- n
20/D 1.523
- Flammpunkt:
- >230 °F
- storage temp.
- Store below +30°C.
- L?slichkeit
- soluble
- Aggregatzustand
- Liquid
- pka
- 13.28±0.20(Predicted)
- Farbe
- Clear colorless to light yellow
- PH
- 12.8 (10g/l, H2O, 20℃)
- Geruch (Odor)
- Unpleasant
- S?ure-Base-Indikators(pH-Indikatoren)
- 12.8 at 10 g/l at 20 °C
- Explosionsgrenze
- 1.1-6.5%(V)
- Wasserl?slichkeit
- soluble
- Sensitive
- Air Sensitive
- BRN
- 508906
- Stabilit?t:
- Stable. Incompatible with strong oxidizing agents, acids, acid chlorides, acid anhydrides.
- InChIKey
- GQHTUMJGOHRCHB-UHFFFAOYSA-N
- CAS Datenbank
- 6674-22-2(CAS DataBase Reference)
- NIST chemische Informationen
- 1,8-diazabicyclo [5.4.0]undec-7-ene(6674-22-2)
- EPA chemische Informationen
- Pyrimido[1,2-a]azepine, 2,3,4,6,7,8,9,10-octahydro- (6674-22-2)
Sicherheit
- Risiko- und Sicherheitserkl?rung
- Gefahreninformationscode (GHS)
| Kennzeichnung gef?hrlicher | C,F | ||
|---|---|---|---|
| R-S?tze: | 22-34-52/53-35-40-37-19-11-67 | ||
| S-S?tze: | 26-36/37/39-45-61-27-16 | ||
| RIDADR | UN 3267 8/PG 2 | ||
| WGK Germany | 2 | ||
| F | 34 | ||
| Selbstentzündungstemperatur | 260 °C | ||
| TSCA | Yes | ||
| HazardClass | 8 | ||
| PackingGroup | II | ||
| HS Code | 29339930 | ||
| Toxizit?t | LD50 orally in Rabbit: 215 - 681 mg/kg |
| Bildanzeige (GHS) |
 
|
|||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alarmwort | Achtung | |||||||||||||||||||||||||||||||||||
| Gefahrenhinweise |
|
|||||||||||||||||||||||||||||||||||
| Sicherheit |
|
1,8-Diazabicyclo(5.4.0)undec-7-en Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R22:Gesundheitssch?dlich beim Verschlucken.R34:Verursacht Ver?tzungen.
R52/53:Sch?dlich für Wasserorganismen, kann in Gew?ssern l?ngerfristig sch?dliche Wirkungen haben.
R35:Verursacht schwere Ver?tzungen.
S-S?tze Betriebsanweisung:
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.S36/37/39:Bei der Arbeit geeignete Schutzkleidung,Schutzhandschuhe und Schutzbrille/Gesichtsschutz tragen.
S45:Bei Unfall oder Unwohlsein sofort Arzt zuziehen (wenn m?glich, dieses Etikett vorzeigen).
S61:Freisetzung in die Umwelt vermeiden. Besondere Anweisungen einholen/Sicherheitsdatenblatt zu Rate ziehen.
S27:Beschmutzte, getr?nkte Kleidung sofort ausziehen.
Beschreibung
DBU is a bicyclic amidine base. It is non-nucleophilic, sterically hindered, tertiary amine base in organic chemistry. It is reported to be superior to amine catalyst in Baylis-Hillman reaction.It promotes the methylation reaction of phenols, indoles and benzimidazoles with dimethyl carbonate under mild conditions.Chemische Eigenschaften
Colorless to yellow liquidOccurrence
Although all commercially available DBU is produced synthetically, it may also be isolated from the sea sponge Niphates digitalis.The biosynthesis of DBU has been proposed to begin with 1,6-hexanedial and 1,3-diaminopropane.Verwenden
DBU may be used:- as catalyst for carboxylic acid esterification with dimethyl carbonate
- in the synthesis of duocarmycin and CC-1065 analogs
- as catalyst in aza-Michael addition and Knovenegal condensation reaction
- as base for dehalogenation of halogenated Diels-Alder adducts and the resulting activated 2,4-dienones were subjected to regio- and stereo-directed Michael additions, using Yamamoto′s reagent (CH3Cu · BF3)
- in a new synthesis of the ABCD ring system of Camptothecin
- DBU may be used as an catalyst for the dissolution and activation of cellulose by a reversible reaction of its hydroxyl groups with carbon dioxide. This dissolved cellulose system can be derivatized to form cellulose mixed esters.
- Used in a new synthesis of the ABCD ring system of Camptothecin.
Allgemeine Beschreibung
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for catalytic efficiency. Click here for more information.Synthese
The synthesis of DBU is as follows:As a base, sodium hydroxide was used at a concentration of 25% by weight and 2.3 times molar equivalent. A stirrer was placed in a 100 mL screw tube, and 11.5 g (50 mmol) of DBU hydrochloride, 8.5 g of toluene and 18.5 g (116 mmol) of 25% sodium hydroxide aqueous solution were charged at room temperature. It was placed on a magnetic stirrer and mixed for 1 hour with stirring. The mixture was separated with a 100 mL separatory funnel to obtain 18.8 g of the upper layer containing DBU and 18.4 g of the lower layer of the aqueous layer. The upper layer portion was analyzed by gas chromatography and contained 31.9% by weight of DBU, and the yield was 6.0 g (39.4 mmol) in a yield of 78.8%.

l?uterung methode
Fractionally distil DBU under vacuum. Also purify it by chromatography on Kieselgel and eluting with CHCl3/EtOH/25% aqueous NH3 (15:5:2) and checking by IR and MS. [Oediger et al. Chem Ber 99 2012 1962, Angew Chem, Int Ed Engl 6 76 1967, Guggisberg et al. Helv Chim Acta 61 1057 1978, Beilstein 23/5 V 271.]1,8-Diazabicyclo(5.4.0)undec-7-en Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
3-Propanolid
4H-AMINO-6-BROMO-2,3-DIHYDROTHIOCHROMEN-1,1-DIOXIDE
5-Phenyl-1,3-oxazole-4-carboxylic acid
Fmoc-8-amino-3,6-dioxaoctanoic acid
1-Isoquinolinecarbonitrile
5-AMINOINDOLE HYDROCHLORIDE
Di(tert-butyl)carbonat
4-CYANOSTYRENE
4-NITROSTYRENE
6-Nitro-1H-indol
5-PHENYL-1,3-OXAZOLE-4-CARBONYL CHLORIDE
(1R,2R)-N-BOC-1-AMINO-2-PHENYLCYCLOPROPANECARBOXYLIC ACID
6-Aminoindole
9-Vinylanthracen
Methyl-1H-pyrrol-1-carboxylat
METSULFURON METHYL
3-ETHYLBENZOPHENONE
N-Boc-N-methyl glycine methyl ester
Phenylpropylether
1,8-Diazabicyclo(5.4.0)undec-7-en Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 750)Lieferanten
| Firmenname | Telefon | Land | Produktkatalog | Edge Rate | |
|---|---|---|---|---|---|
| CD Chemical Group Limited | +8615986615575 |
info@codchem.com | China | 20342 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-86-5926051114 +8615060885618 |
sales@amoychem.com | China | 6369 | 58 |
| Nanjing Sinoda Biological Technology Co., Ltd | +8613401983379 |
sales@njmcn.cn | CHINA | 74 | 58 |
| Shandong Believe Chemical PTE.LTD | +86-18553607090 +8618553607090 |
1093908296@qq.com | China | 1664 | 58 |
| Hebei Chuanghai Biotechnology Co., Ltd | +8617732866630 |
abby@chuanghaibio.com | China | 8773 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5251 | 58 |
| Zibo Hangyu Biotechnology Development Co., Ltd | +86-0533-2185556 +8615965530500 |
nickzhang@hangyubiotech.com | China | 11000 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 20202 | 58 |
| Shandong Juchuang Chemical Co., LTD | +16837774622 |
admin@juchuangchem.com | China | 295 | 58 |
| airuikechemical co., ltd. | +86-18353166132 |
sales02@airuikechemical.com | China | 983 | 58 |
6674-22-2(1,8-Diazabicyclo(5.4.0)undec-7-en)Verwandte Suche:
Tosylazid
Hexamethylen-1,6-diisocyanat
Non-1-en
1,8-Diazabicyclo(5.4.0)undec-7-en
1-Benzyl-2,3,4,6,7,8,9,10-octahydropyrimido[1,2-a]azepiniumchlorid
2,3,4,6,7,8,9,10-Octahydropyrimido[1,2-a]azepinium-(p-tolylsulfonat)
- 1,8-DIAZABICYCLO[5.4.0]UNDEC-7-ENE
- 1,8-DIAZABICYCLO[5.4.0]UNDEC-7-ENE (1,5-5)
- 1,8-DIAZEBICYCLO[5.4.0]UNDEC-7-ENE
- 1,8-Diazabicyclo[5.4.0]undecane-7-ene
- 1,8-Diazabicyclo(5 x 4 x 0)undec-7-ene
- Pyrimido[1,2-a]azep
- 1,8-Diazabicyclo[5.4.]undec-7-ene
- 1,8-Diazabicyclo[5.4.0]undec-7-ene, 98% 100GR
- 1,8-Diazabicyclo[5.4.0]undec-7-ene, 98% 500GR
- 1,8-Diazabicyclo[5.4.0]undec-7-ene, 98% 5GR
- 1,8-Diazabicyclo[5.4.0]undec-7-ene,2,3,4,6,7,8,9,10-Octahydropyrimidol[1,2-a]azepine, DBU
- 8-Diazabicyclo[5.4.0]undec-7-ene (DBU)
- Lupragen N700 (Diazabicycloundecene)
- DBU 2,3,4,6,7,8,9,10-Octahydropyrimido[1,2-a]azepine
- 1,8-Diazabicyclo[5.4.0]undec-7-ene, 2,3,4,6,7,8,9,10-OctahydropyriMido[1,2-a]azepine
- 1,8-DIAZABICYCLO[5.4.0]UNDEC-7-ENE FOR S
- 1,8- twoazabicyclo[5.4.0] eleven carbon-7- ene
- 1,8-Diazabicyclo[5.4.0]undec-7-ene puriss., >=99.0% (GC)
- 1,8-Diazabicyclo[5.4.0]-7-undecene, 98.0%(GC&T)
- Is a
- 1,8-Diazabicyclo[5.4.0]undec-7-ene solution 1 M in ethyl acetate
- 1,8-Diazabicyclo[5.4.0]undec-7-ene solution
- 1,5-Diaza(5,4,0)undec-5-ene
- 1,5-Diazabicyclo[5.4.0]-5-undecene
- 2,3,4,6,7,8,9,10-Octahydropyrimido(1,2-alpha)azepine
- 2-a]azepine,2,3,4,6,7,8,9,10-octahydro-pyrimido[
- 2-a]azepine,2,3,4,6,7,8,9,10-octahydro-Pyrimido[1
- Pyrimido[1,2-a]azepine,2,3,4,6,7,8,9,10-octahydro-
- 2,3,4,6,7,8,9,1-Octahydropyrimido[1,2-a]azepine
- 2,3,4,6,7,8,9,10-Octahydropyrimido[1,2-a]azepine, DBU
- 1,8-diazabicyclo[5,4,0]-undecen-(7)
- Dicyclo[5,4,0]-1,8-Diazole-7-Nonylene
- 2,3,4,6,7,8,9,10-Octahydropyrimidol[1,2-A]Aze
- 1,8-Diazabicyclo[5,4,0]undec-7
- 1,8-Diaza-7-bicyclo[5.4.0]undecene.
- 2,3,4,6,7,8,9,10-OCTAHYDROPYRIMIDOL[1,2-A]AZEPINE
- 2,3,4,6,7,8,9,10-OCTA-HYDRO-PYRIMIDOL[1,2-ALPHA]AZEPINE
- LUPRAGEN(R) N 700
- POLYCAT(R) 18
- POLYCAT(R) 48
- OCTAHYDROPYRIMIDO(1,2:A)AZEPINE
- 1,8-DIAZABICYCLO [5.4.0] UNDEC-7-ENE (AKA) DBU
- 1,8-Diazabicyclo5.4.0üundec-7-ene, 99%
- 2,3,4,6,7,8,9,10-Octahydropyrimidol[1,2-a]azepine, DBU
- U-CAT SA 851
- 1,8-Diazabicyclo[5.4.0]undec-7-ene ,98%
- Diazabicycloundecene (DBU)
- 1,8-Diazabicyclo[5.4.0]undec-7-ene, 1 M in ethyl acetate, J&KSeal
- 1,8-Diazabicyclo[5.4.0]undec-7-ene, 1 M in THF, J&KSeal
- Baloxavir Impurity 44
- DBU (liquid) 1,8-Diazabicyclo[5.4.0]undec-7-ene
- o[5.4.0]-7-undecene
- Tofacitinib Impurity 164
- Cycloisopropylidene malonate
- 1.0mol/l 1,8-Diazabicyclo[5.4.0]undec-7-ene Tetrahydrofuran Solution
- 1,8-Diazabicyclo[5.4.0]-7-undecene >
- DBU 1,8-Diazabicyclo[5.4.0]undec-7-ene,98%
- Lupragen N700