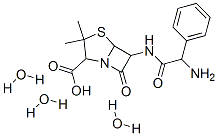
Ampicillin
|
|
Ampicillin Eigenschaften
- Schmelzpunkt:
- 208 °C (dec.)(lit.)
- Siedepunkt:
- 684℃
- Brechungsindex
- 265 ° (C=0.1, H2O)
- Flammpunkt:
- 87 °C
- storage temp.
- Sealed in dry,2-8°C
- L?slichkeit
- NH4OH 1 M: 50 mg/mL, clear, colorless
- Aggregatzustand
- solid
- Farbe
- white to off-white
- pka
- 2.5 (COOH)(at 25℃)
- Wasserl?slichkeit
- 0.1-1 g/100 mL at 21 ºC
- Merck
- 14,586
- BRN
- 5399534
- Stabilit?t:
- Hygroscopic
- InChIKey
- RXDALBZNGVATNY-CWLIKTDRSA-N
- CAS Datenbank
- 7177-48-2(CAS DataBase Reference)
- EPA chemische Informationen
- Ampicillin trihydrate (7177-48-2)
Sicherheit
- Risiko- und Sicherheitserkl?rung
- Gefahreninformationscode (GHS)
| Kennzeichnung gef?hrlicher | Xn,Xi | ||
|---|---|---|---|
| R-S?tze: | 36/37/38-42/43 | ||
| S-S?tze: | 22-26-36/37-36-45-23 | ||
| WGK Germany | 2 | ||
| RTECS-Nr. | XH8425000 | ||
| F | 3-10 | ||
| HS Code | 29411020 | ||
| Toxizit?t | LD50 orl-rat: 10 g/kg ANTBAL 20,653,75 |
| Bildanzeige (GHS) |

|
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alarmwort | Achtung | |||||||||||||||||||||
| Gefahrenhinweise |
|
|||||||||||||||||||||
| Sicherheit |
|
Ampicillin Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R36/37/38:Reizt die Augen, die Atmungsorgane und die Haut.R42/43:Sensibilisierung durch Einatmen und Hautkontakt m?glich.
S-S?tze Betriebsanweisung:
S22:Staub nicht einatmen.S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.
S36/37:Bei der Arbeit geeignete Schutzhandschuhe und Schutzkleidung tragen.
S36:DE: Bei der Arbeit geeignete Schutzkleidung tragen.
Chemische Eigenschaften
Ampicillin in anhydrous form occurs as crystals.Verwenden
Commonly used to select for ampicillin resistance in mutated and transformed cellsIndications
Ampicillin may also be helpful in certain patients, particularly pregnant women with acne, for whom the use of tetracycline, erythromycin, and minocycline should be avoided. In resistant acne patients, culture may reveal a gram-negative bacteria responsive to ampicillin.Antimicrobial activity
Ampicillin is slightly less active than benzylpenicillin against most Gram-positive bacteria but is more active against E. faecalis. MRSA and strains of Str. pneumoniae with reduced susceptibility to benzylpenicillin are resistant. Most group D streptococci, anaerobic Gram-positive cocci and bacilli, including L. monocytogenes, Actinomyces spp. and Arachnia spp., are susceptible. Mycobacteria and nocardia are resistant.Ampicillin has similar activity to benzylpenicillin against N. gonorrhoeae, N. meningitidis and Mor. catarrhalis. It is 2–8 times more active than benzylpenicillin against H. influenzae and many Enterobacteriaceae, but β-lactamase-producing strains are resistant. Pseudomonas spp. are resistant, but Bordetella, Brucella, Legionella and Campylobacter spp. are often susceptible. Certain Gram-negative anaerobes such as Prevotella melaninogenica and Fusobacterium spp. are susceptible, but B. fragilis is resistant, as are mycoplasmas and rickettsiae.
Activity against molecular class A β-lactamase-producing strains of staphylococci, gonococci, H. influenzae, Mor. catarrhalis, certain Enterobacteriaceae and B. fragilis is enhanced by the presence of β-lactamase inhibitors, specifically clavulanic acid.
Its bactericidal activity resembles that of benzylpenicillin. Bactericidal synergy occurs with aminoglycosides against E. faecalis and many enterobacteria, and with mecillinam against a number of ampicillin-resistant enterobacteria.
Acquired resistance
β-Lactamase-producing pathogens, including most clinical isolates of Staph. aureus, are resistant. Strains of pneumococci, enterococci, gonococci and H. influenzae with altered PBPs have reduced susceptibility to ampicillin. Isolates of N. gonorrhoeae and H. influenzae with a TEM plasmid- mediated β-lactamase (which are more common) are fully resistant. Resistance among H. influenzae is often linked with resistance to chloramphenicol, erythromycin or tetracycline, due to plasmid-encoded resistance markers that are co-transferred with the gene for the TEM enzyme. However, at least 70% of current H. influenzae isolates remain susceptible to ampicillin worldwide.The widespread use of ampicillin and other aminopenicillins has led to resistance becoming common in formerly susceptible species of enteric pathogens as a result of the widespread dissemination of plasmid-mediated β-lactamases. Surveillance data from North America and Europe indicate less than 50% susceptibility to ampicillin in Esch. coli. At least 90% of current isolates of Mor. catarrhalis are β-lactamaseproducing strains. Ampicillin-resistant strains of salmonellae, notably S. enterica serotypes Typhi and Typhimurium (many of which are also resistant to chloramphenicol, sulfonamides and tetracyclines) present a serious problem in Africa, Asia and South America. Multiresistant strains of shigellae also predominate in many parts of the world.
Allgemeine Beschreibung
Odorless white microcrystalline powder with a bitter taste. A 0.25% solution in water has a pH of 3.5 to 5.5.Air & Water Reaktionen
Slightly soluble in water.Reaktivit?t anzeigen
Ampicillin absorbs insignificant amounts of moisture at 77° F and relative humidities up to approximately 80%, but under damper conditions Ampicillin absorbs significant amounts. A pH-rate profile reveals specific-acid- and specific-base- catalyzed hydrolysis. The pH of maximum stability is 5.8.Brandgefahr
Flash point data for Ampicillin are not available; however, Ampicillin is probably combustible.Kontakt-Allergie
Ampicillin caused contact dermatitis in a nurse also sensitized to amoxicillin (with tolerance to oral phenoxymethylpenicillin) and in a pharmaceutical factory worker. Systemic drug reactions are common. Crossreactivity is regular with ampicillin and can occur with other penicillins.Clinical Use
Isolates should be tested for susceptibility before use, especially for serious infections. For oral therapy, amoxicillin is preferable to ampicillin.Urinary tract infections
Bacterial meningitis
Respiratory tract infections
Gastrointestinal infections, including typhoid fever and bacillary dysentery Enterococcal endocarditis and septicemia (in combination with an aminoglycoside)
Listeriosis (in combination with an aminoglycoside)
Sicherheitsprofil
Mildly toxic by ingestion. An experimental teratogen. Other experimental reproductive effects. When heated to decomposition it emits toxic fumes of SO,xand NOx.m?gliche Exposition
Used as an antibiotic.Versand/Shipping
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.Inkompatibilit?ten
May be incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.Waste disposal
It is inappropriate and possibly dangerous to the environment to dispose of expired or waste pharmaceuticals by flushing them down the toilet or discarding them to the trash. Household quantities of expired or waste pharmaceuticals may be mixed with wet cat litter or coffee grounds, double-bagged in plastic, discard in trash. Larger quantities shall carefully take into consideration applicable DEA, EPA, and FDA regulations. If possible return the pharmaceutical to the manufacturer for proper disposal being careful to properly label and securely package the material. Alternatively, the waste pharmaceutical shall be labeled, securely packaged and transported by a state licensed medical waste contractor to dispose by burial in a licensed hazardous or toxic waste landfill or incinerator.Ampicillin Upstream-Materialien And Downstream Produkte
Upstream-Materialien
N,N-Diethylethanamin
Pivaloylchlorid
SECONDARYAMINES
Urea, N,N-bis(trimethylsilyl)-
6-Aminopenicillansure
Chlortrimethylsilan
Dichlormethan
Downstream Produkte
Ampicillin Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 714)Lieferanten
| Firmenname | Telefon | Land | Produktkatalog | Edge Rate | |
|---|---|---|---|---|---|
| Wuhan Fortuna Chemical Co., Ltd | +86-027-59207850 +86-13986145403; |
info@fortunachem.com | China | 5948 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +86-17396673057 |
linda@tnjone.com | China | 1143 | 58 |
| Hebei Longbang Technology Co., LTD | +86-18633929156 |
admin@hblongbang.com | China | 972 | 58 |
| Shaanxi Xianhe Biotech Co., Ltd | +86-17709210191; +8617709210191 |
Jerry@xhobio.com | China | 884 | 58 |
| Aurora Industry Co., Ltd. | +86-13591747876 |
alex1_auco@126.com | China | 300 | 58 |
| Hangzhou Verychem Science And Technology Co.Ltd | +86-86-57188162785 13606544505 |
lucy@verychem.com | China | 1114 | 58 |
| Capot Chemical Co.,Ltd. | +8613336195806 |
sales@capot.com | China | 29731 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21622 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29855 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-86-5926051114 +8615060885618 |
sales@amoychem.com | China | 6369 | 58 |
7177-48-2()Verwandte Suche:
N,N-Dimethylformamid
N,N-Dimethyl-phenylendiamin (p)
Dimethylcarbonat
Dimethylsulfat
Ethan
Dimethylfumarat
Dimethyl ether
Paracetamol
Dimethylsulfoxid
Ampicillin sodium/sullbactam sodium
Amoxicillin
Natrium-[2S-[2α,5α,6β(S*)]]-6-(aminophenylacetamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptan-2-carboxylat
Ampicillin
Pivampicillin
Azlocillin
Natrium-[2S-[2α,5α,6β(S*)]]-3,3-dimethyl-7-oxo-6-[[[[(2-oxoimidazolidin-1-yl)carbonyl]amino]phenylacetyl]amino]-4-thia-1-azabicyclo[3.2.0]heptan-2-carboxylat
Natrium-[2S-[2α,5α,6β(S*)]]-6-[[amino(4-hydroxyphenyl)acetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptan-2-carboxylat
Mezlocillin
- amcap
- aminobenzylpenicillintrihydrate
- amperil
- ampichel
- ampinova
- ro-ampen
- trihydrate,d-(-)-do)-3-dimethyl-7-oxo-
- AMP TABS(TM)
- ALPHA-AMINOBENZYLPENICILLIN, TRIHYDRATE
- (2s,5r,6r)-6-[(r)-2-amino-2-phenylacetamido]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid
- (2S,5R,6R)-6-((R)-2-AMINO-2-PHENYL-ACETYLAMINO)-3,3-DIMETHYL-7-OXO-4-THIA-1-AZA-BICYCLO[3.2.0]HEPTANE-2-CARBOXYLIC ACID
- 6-[D-ALPHA-AMINOPHENYLACETAMIDO]PENICILLANIC ACID TRIHYDRATE
- d-(-)-6-(2-amino-2-phenylacetamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid trihydrate
- D(-)-ALPHA-AMINOBENZYLPENICILLIN TRIHYDRATE
- ampicillin,cilleral,principen
- AMPICILLIN TRIHYDRATE VETRANAL, 250
- 4-Thia-1-azabicyclo(3.2.0)heptane-2-carboxylic acid, 6-(2-amino-2-phenylacetamido)- 3,3-dimethyl-7-oxo-, trihydrate, D- (-)
- Ampicillin trihydrate,D-()-α-Aminobenzylpenicillin
- Ampicillin Trihydrate (200 mg)
- AMPICILLIN CRYST.RESEARCH GRADE
- AMpicillin Trihydrate CoMpacted / Powder
- 4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 6-[[(2R)-2-amino-2-phenylacetyl]amino]-3,3-dimethyl-7-oxo-, hydrate (1:3), (2S,5R,6R)-
- 6-{[Amino(phenyl)acetyl]amino}-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxyl
- amplin
- cymbi
- divercillin
- lifeampil
- morepen
- nci-c56086
- pensyn
- AMPICILLIN TRIHYDRATE CRYSTALLINE
- AMPICILLIN TRIHYDRAT, USP*
- AMPICILLIN TRIHYDRATE USP
- AMPICILLIN (ANHYDROUS) USP(CRM STANDARD)
- AMPICILLIN (ANHYDROUS) WHO(CRM STANDARD)
- AMPICILLIN IMP. C (EP):*) AMPICILLIN DIKETOPIPERAZINE MM(CRM STANDARD)
- AMPICILLIN IRRADIATED TISSUE CULTURE GRADE
- AMPICILLIN TRIHYDRATE EPA(CRM STANDARD)
- (6-D-[-]-α-Aminophenylacetamido) penicillanic acid trihydrate
- 4-Thia-1-azabicyclo3.2.0heptane-2-carboxylic acid, 6-(2R)-aminophenylacetylamino-3,3-dimethyl-7-oxo-, trihydrate, (2S,5R,6R)-
- AMPICILLIN TRIHYDRATE MM(CRM STANDARD)
- AMPICILLIN TRIHYDRATE USP(CRM STANDARD)
- AMPICILLIN TRIHYDRATE WHO(CRM STANDARD)
- AmpicillinAnhydrousAmpicillinAnhydrousBp/Usp
- AmpicillinTrihydrateUspGrade
- AmpicillinTrihydrateAmpicillinTrihydrateBp/Usp
- AMPICILLIN 3-HYDRATE, PHARMA
- AMPICILLIN 3-HYDRATE
- AMPICILLINTRIHYDRATE,MICRONIZED,USP
- [D-(-)-α-Aminobenzyl]penicillin trihydrate
- 4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 6-(2-amino-2-phenylacetamido)-3,3-dimethyl-7-oxo-, trihydrate, D-(-)- (8CI)
- 4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 6-[(aminophenylacetyl)amino]-3,3-dimethyl-7-oxo-, trihydrate, [2S-[2α,5α,6β(S*)]]-
- Ampicillin
- (2S,5R,6R)-6-[[(2R)-2-amino-2-phenyl-acetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid triydrate
- Piperacillin Impurity A
- Ampicillin Impurity
- Ampicillin Trihydrate>
- Ampicillin trihydrate CRS