| Identification | More | [Name]
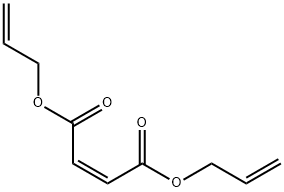
DIALLYL MALEATE | [CAS]
999-21-3 | [Synonyms]
BUTENEDIOIC ACID DIALLYL ESTER
DI-(2-PROPENYL)MALEATE
DIALLYL MALEATE
DIALLYL MALLEATE
MALEIC ACID DIALLYL ESTER
2-Butenedioic acid (Z)-, di-2-propenyl ester
2-Butenedioicacid(Z)-,di-2-propenylester
Diallyl (2Z)-2-butenedioate
Diallylcis-butenedioate
Diallylester kyseliny maleinove
diallylesterkyselinymaleinove
dlallylmaleate
Sipomer dam
sipomerdam
DIALLYL MALEATE, TECH., 93%
DIALLYL MALEATE, MONOMER, STAB.
MALEIC ACID DIALLYL ESTER (STABILIZED WITH HQ) 98+%
Diallylmaleate,95%
2-Butenedioic acid (2Z)-, di-2-propenyl ester
Diallyl Maleate (stabilized with HQ) | [EINECS(EC#)]
213-658-0 | [Molecular Formula]
C10H12O4 | [MDL Number]
MFCD00010763 | [Molecular Weight]
196.2 | [MOL File]
999-21-3.mol |
| Chemical Properties | Back Directory | [Melting point ]
−47 °C(lit.)
| [Boiling point ]
106-116 °C4 mm Hg
| [density ]
1.074 g/mL at 20 °C(lit.)
| [vapor density ]
6.6 (vs air)
| [vapor pressure ]
4 mm Hg ( 25 °C)
| [refractive index ]
n20/D 1.469(lit.)
| [Fp ]
>230 °F
| [storage temp. ]
2-8°C | [form ]
clear liquid | [color ]
Colorless to Almost colorless | [Odor]
pungent odor | [Water Solubility ]
Soluble in water 151.2 mg/L @ 25°C. | [BRN ]
1725954 | [InChIKey]
ZPOLOEWJWXZUSP-WAYWQWQTSA-N | [LogP]
2.126 (est) | [CAS DataBase Reference]
999-21-3(CAS DataBase Reference) | [EPA Substance Registry System]
Diallyl maleate (999-21-3) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R21/22:Harmful in contact with skin and if swallowed .
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37:Wear suitable protective clothing and gloves . | [RIDADR ]
UN 2810 6.1/PG 3
| [WGK Germany ]
3
| [RTECS ]
ON0700000
| [TSCA ]
Yes | [HazardClass ]
6.1 | [PackingGroup ]
III | [HS Code ]
29171900 | [Safety Profile]
Poison by ingestion and
intraperitoneal routes. Moderately toxic by
skin contact. A skin and eye irritant. When
heated to decomposition it emits acrid
smoke and irritating fumes. See also ALLYL
COMPOUNDS and ESTERS. |
| Hazard Information | Back Directory | [Chemical Properties]
Colorless liquid | [Uses]
An agent for the promotion of branching in emulsion polymers | [Preparation]
Diallyl maleate is obtained by the sulfuric acid-catalyzed esterification of maleic anhydride with propyl alcohol. | [Synthesis]
A typical procedure for the synthesis of diallyl maleate from maleic anhydride and acryl alcohol is as follows: maleic anhydride (1 mmol, 0.15 g), acryl alcohol (5 mmol, 0.46 mL, 0.37 g), and HFDAIL as a catalyst (10 mol/mol of maleic anhydride, 0.05 g) were charged into a 50 mL round-bottomed flask equipped with a Dean-Stark device, reflux condenser, and magnetic stirrer in a 50 mL round bottom flask. The reaction mixture was stirred at 125 °C for 7 hours. The progress of the reaction was monitored by thin layer chromatography (TLC) using ethyl acetate/hexane (2:8) as eluent. When TLC showed that the reaction was complete, the isolated product (diallyl maleate) was obtained by extraction with ethyl acetate (3 x 5 mL) and evaporation of ethyl acetate under reduced pressure to give the target product as a yellow oily liquid in 95% yield (0.26 g, boiling point = 340 °C). The resulting product was dried under vacuum at 70 °C for 5 hours. The viscous ionic liquid can be directly reused without further treatment after removing water under vacuum at 80 °C for 5 h. The product was dried under vacuum for 5 h at 70 °C. | [References]
[1] Journal of Molecular Liquids, 2017, vol. 227, p. 153 - 160
[2] Patent: US2249768, 1939,
[3] Sci. Ind. Osaka, 1958, vol. 32, p. 228
[4] Chem.Abstr., 1959, p. 7007
[5] Patent: US2249768, 1939, |
|
|