| Identification | Back Directory | [Name]
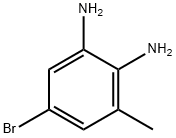
5-BROMO-3-METHYL-BENZENE-1,2-DIAMINE | [CAS]
76153-06-5 | [Synonyms]
5-BROMO-3-METHYL-1,2-BENZENEDIAMINE
5-Bromo-3-methyl-1,2-diaminobenzene
5-BROMO-3-METHYL-BENZENE-1,2-DIAMINE
1,2-Benzenediamine, 5-bromo-3-methyl- | [Molecular Formula]
C7H9BrN2 | [MDL Number]
MFCD06411036 | [MOL File]
76153-06-5.mol | [Molecular Weight]
201.06 |
| Chemical Properties | Back Directory | [Melting point ]
63 °C | [Boiling point ]
298.8±35.0 °C(Predicted) | [density ]
1.589±0.06 g/cm3(Predicted) | [storage temp. ]
Keep in dark place,Inert atmosphere,2-8°C | [pka]
3.31±0.10(Predicted) | [Appearance]
Brown to dark brown Solid | [InChI]
InChI=1S/C7H9BrN2/c1-4-2-5(8)3-6(9)7(4)10/h2-3H,9-10H2,1H3 | [InChIKey]
UOFSLKHZOPVGHG-UHFFFAOYSA-N | [SMILES]
C1(N)=CC(Br)=CC(C)=C1N | [CAS DataBase Reference]
76153-06-5 |
| Hazard Information | Back Directory | [Uses]
5-Bromo-3-methylbenzene-1,2-diamine is used as an intermediate in organic synthesis. | [Synthesis]
Step 1: Stannous chloride (SnCl2, 5.0 g, 26.1 mmol) was added to an ethanol (EtOH, 20 mL) suspension of 4-bromo-2-methyl-6-nitroaniline (2.0 g, 8.7 mmol). The reaction mixture was heated to reflux for 14 h, followed by cooling to room temperature and vacuum concentration. The residue was diluted with ethyl acetate (EtOAc, 100 mL) and partitioned between saturated aqueous sodium bicarbonate (NaHCO3) solution (200 mL) and water. The resulting slurry was filtered through a Celite pad and the wet filter cake was washed with ethyl acetate (3 x 50 mL). The filtrate was washed sequentially with saturated aqueous sodium bicarbonate, water, and brine, dried over anhydrous magnesium sulfate (MgSO4), filtered, and concentrated in vacuum to give 1.27 g (72% yield) of 5-bromo-3-methylbenzene-1,2-diamine (72) as a yellow oil: mass spectrum (ESI) m/z = 201.1 [M + 1]+. | [References]
[1] Patent: WO2013/26914, 2013, A1. Location in patent: Page/Page column 130
[2] Bioorganic and Medicinal Chemistry Letters, 2007, vol. 17, # 11, p. 3072 - 3076
[3] Patent: US2005/54655, 2005, A1. Location in patent: Page/Page column 15-16
[4] Patent: US2004/44203, 2004, A1. Location in patent: Page 28
[5] Chemische Berichte, 1884, vol. 17, p. 776 |
|
|