| Identification | More | [Name]
2-Amino-3-pyridinecarboxaldehyde | [CAS]
7521-41-7 | [Synonyms]
2-AMINO-3-FORMYLPYRIDINE
2-AMINO-3-PYRIDINECARBOXALDEHYDE
2-AMINONICOTINALDEHYDE
2-AMINO-PYRIDIN-3-CARBALDEHYDE
2-AMINO-PYRIDINE-3-CARBALDEHYDE
2-AMINOPYRIDINE-3-CARBOXALDEHYDE
3-PYRIDINECARBOXALDEHYDE, 2-AMINO-
2-Aminopyridine-3-carboxaldehyde, 2-Aminonicotinaldehyde
2-Amino-3-Pyridine Formaldehyde
2-Amino-3-formylpyridine, 2-Aminonicotinaldehyde
2-Aminopyridine-3-carboxaldehyde 99%
2-AMINO-3-PYRIDINECARBOXALDEHYDE 2-AMINONICOTINALDEHYDE
2-Aminopyridine-3-carboxyaldehyde
3-formylpyridin-2-amine | [EINECS(EC#)]
626-730-5 | [Molecular Formula]
C6H6N2O | [MDL Number]
MFCD01830382 | [Molecular Weight]
122.12 | [MOL File]
7521-41-7.mol |
| Chemical Properties | Back Directory | [Appearance]
yellow crystals | [Melting point ]
98-102 °C (lit.) | [Boiling point ]
290.7±25.0 °C(Predicted) | [density ]
1.264±0.06 g/cm3(Predicted) | [Fp ]
>300℃ | [storage temp. ]
Refrigerator (+4°C) | [solubility ]
Soluble in Ether, Ethyl acetate, Chloroform (Slightly), Methanol (Slightly) | [form ]
Powder, Crystals and/or Chunks | [pka]
4.99±0.36(Predicted) | [color ]
Yellow to light brown | [Water Solubility ]
insoluble | [Sensitive ]
Air Sensitive | [Detection Methods]
HPLC | [BRN ]
109598 | [InChI]
InChI=1S/C6H6N2O/c7-6-5(4-9)2-1-3-8-6/h1-4H,(H2,7,8) | [InChIKey]
NXMFJCRMSDRXLD-UHFFFAOYSA-N | [SMILES]
C1(N)=NC=CC=C1C=O | [CAS DataBase Reference]
7521-41-7(CAS DataBase Reference) | [Storage Precautions]
Air sensitive |
| Safety Data | Back Directory | [Hazard Codes ]
Xi,Xn | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin .
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection . | [WGK Germany ]
3 | [Hazard Note ]
Irritant | [HazardClass ]
IRRITANT | [PackingGroup ]
II | [HS Code ]
29339900 |
| Hazard Information | Back Directory | [Chemical Properties]
yellow crystals | [Uses]
2-Aminonicotinaldehyde is commonly employed as a starting material for a wide variety of N-heterocyclic compounds (e.g. β-nicotyrine [N445000]) . 2-Aminonicotinaldehyde is also used as a reagent to synthesize hydrazones (e.g.thionaphthenquinone 3-hydrazone [T367720]), which possess antituburcular properties. | [Preparation]
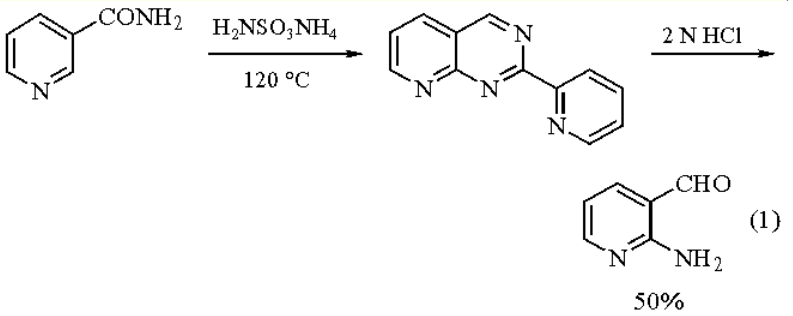
Preparative Methods of 2-Amino-3-pyridinecarboxaldehyde: the reagent is best when freshly prepared. A multistep procedure starting from 2-amino-3-
picoline has been improved upon by a simple two-step approach. Sulfamation of nicotinamide with ammonium
sulfamate (both commercially available) provides 2-(3′-pyridyl)pyrido[2,3-d]pyridimine which may be
hydrolyzed in 2 N HCl to provide the reagent (eq 1)[1]. Substituted derivatives of the reagent may be prepared via
a similar intermediate.
 | [Synthesis]
General procedure for the synthesis of 2-amino-3-pyridinecarboxaldehyde from the compound (CAS: 7521-27-9): first, a 2 mol/L hydrochloric acid solution was prepared. The dried feedstock was transferred to a 50 mL single neck flask and about 30 mL of the above hydrochloric acid solution was added. The mixture was heated to reflux for 4 hours and subsequently cooled to room temperature. The pH of the reaction mixture was adjusted to 7-8 using 10 mol/L sodium hydroxide solution. 5 extractions were carried out with ether and the organic phases were combined and dried over anhydrous potassium carbonate. The solvent was removed by rotary evaporation to give a yellow solid product. Finally, it was purified by column chromatography (unfolding agent ratio VD TM/VEIQH = 30:1) and dried at low temperature to obtain pure 2-amino-3-pyridinecarboxaldehyde in light yellow color in 30% yield. | [storage]
2-Amino-3-pyridinecarboxaldehyde is somewhat more stable than 2-Aminobenzaldehyde and may
be stored for up to several months at 0 °C under an inert atmosphere. Sublimation is a convenient method for the
separation of higher oligomers which may accumulate during storage. Use in a fume hood. | [References]
1. (a) Caluwe, P. T 1980, 36, 2359. (b) Cheng, C.-C.; Yan, S.-J. OR 1982, 28, 37. (c) Thummel, R. P. SL 1992, 1. |
|
|