| Identification | Back Directory | [Name]
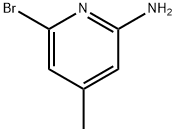
2-Bromoo-4-methyl-6-aminopyridine | [CAS]
73895-98-4 | [Synonyms]
6-Bromo-4-Methylpyridin-2-amine
2-Amino-4-methyl-6-bromopyridine
2-PyridinaMine,6-broMo-4-Methyl-
6-AMino-2-broMo-4-Methylpyridine
2-Bromo-4-Methyl-6-Aminopyridine
2-Amine-6-bromo-4-methylpyridine
2-Bromoo-4-methyl-6-aminopyridine
6-bromo-4-methyl-pyridin-2-ylamine | [Molecular Formula]
C6H7BrN2 | [MDL Number]
MFCD11036206 | [MOL File]
73895-98-4.mol | [Molecular Weight]
187.04 |
| Chemical Properties | Back Directory | [Melting point ]
115 °C | [Boiling point ]
293.7±35.0 °C(Predicted) | [density ]
1.593±0.06 g/cm3(Predicted) | [storage temp. ]
under inert gas (nitrogen or Argon) at 2–8 °C | [form ]
solid | [pka]
3.39±0.35(Predicted) | [color ]
White to off white |
| Hazard Information | Back Directory | [Synthesis]
The general procedure for the synthesis of 6-bromo-4-methylpyridin-2-amine using 3-hydroxy-3-methylglutaronitrile as starting material was as follows: 3-hydroxy-3-methylglutaronitrile was slowly added to a 33% solution of hydrogen bromide in glacial acetic acid under ice bath conditions. The reaction mixture was stirred at room temperature for 3 days. Upon completion of the reaction, the solvent was removed by distillation under reduced pressure. Subsequently, the pH of the residue was adjusted to 12 with 10 M aqueous sodium hydroxide. three extractions of the basic aqueous solution were performed with ethyl acetate (100 mL). The organic phases were combined and the solvent was removed by distillation under reduced pressure to give 6-amino-2-bromo-4-methylpyridine (56 g, 66% yield) as colorless crystals with a melting point of 99°C. The residue was extracted with ethyl acetate (100 mL). | [References]
[1] Patent: US5374604, 1994, A
[2] Bioorganic and Medicinal Chemistry Letters, 2006, vol. 16, # 7, p. 1892 - 1897 |
|
| Company Name: |
Energy Chemical
|
| Tel: |
021-021-58432009 400-005-6266 |
| Website: |
http://www.energy-chemical.com |
|