| Identification | More | [Name]
1,4-Cyclohexanedione mono(2,2-dimethyltrimethylene ketal) | [CAS]
69225-59-8 | [Synonyms]
1,4-CYCLOHEXANEDIONE MONO-2,2-DIMETHYLTRIMETHYLENE ACETAL
1,4-CYCLOHEXANEDIONE MONO-2,2-DIMETHYLTRIMETHYLENE KETAL
1,4-CYCLOHEXANEDIONE MONONEOPENTYL KETAL
3,3-DIMETHYL-1,5-DIOXASPIRO[5.5]UNDECAN-9-ONE
1,4-cyclohexanedione mono-2,2-dimethyl-trimethyle
3,3-Dimethyl-1,5-dioxaspiro[5.5]undecan-8-one | [EINECS(EC#)]
273-918-4 | [Molecular Formula]
C11H18O3 | [MDL Number]
MFCD00006652 | [Molecular Weight]
198.26 | [MOL File]
69225-59-8.mol |
| Chemical Properties | Back Directory | [Appearance]
White to yellowish powder | [Melting point ]
45-50 °C(lit.) | [Boiling point ]
70 °C(Press: 0.05 Torr) | [density ]
1.08±0.1 g/cm3(Predicted) | [Fp ]
>230 °F
| [storage temp. ]
Sealed in dry,Room Temperature | [solubility ]
Acetone, Dichloromethane, Ethyl Acetate, Methanol, | [form ]
Solid | [color ]
White | [BRN ]
3541930 | [InChI]
InChI=1S/C11H18O3/c1-10(2)7-13-11(14-8-10)5-3-9(12)4-6-11/h3-8H2,1-2H3 | [InChIKey]
COKVDTKAWIFNTH-UHFFFAOYSA-N | [SMILES]
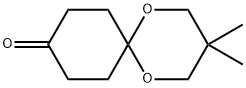
O1C2(CCC(=O)CC2)OCC(C)(C)C1 | [CAS DataBase Reference]
69225-59-8(CAS DataBase Reference) |
| Safety Data | Back Directory | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S24/25:Avoid contact with skin and eyes . | [WGK Germany ]
3
| [HS Code ]
29329990 |
| Hazard Information | Back Directory | [Chemical Properties]
White to yellowish powder | [Uses]
1,4-Cyclohexanedione mono(2,2-dimethyltrimethylene ketal)is a reagent used in the preparation of carbazole derivatives.
| [Synthesis Reference(s)]
Synthetic Communications, 14, p. 39, 1984 DOI: 10.1080/00397918408060862 | [Synthesis]
The general procedure for the synthesis of 3,3-dimethyl-1,5-dioxaspiro[5.5]undecan-9-one using the compound (CAS: 69825-15-6) as starting material was as follows: first, 6.1 mg (0.02 mmol) of sodium 2-iodobenzenesulfonate, 0.49 g (0.8 mmol) of Oxone? (registered trademark) powder, 0.5 g (3.5 mmol) anhydrous sodium sulfate and 200 mg (1 mmol) 3,3-dimethyl-1,5-dioxaspiro[5.5]undecan-9-ol were added to 5 mL of ethyl acetate. Subsequently, the reaction mixture was heated and stirred at 70°C for 8 hours under nitrogen protection. Upon completion of the reaction, post-processing was carried out according to the method of Example 1, resulting in the target product 3,3-dimethyl-1,5-dioxaspiro[5.5]undecan-9-one. The yield of the compound was determined to be 91%, as shown in Table 2. | [References]
[1] Patent: EP2085373, 2009, A1. Location in patent: Page/Page column 12; 14-15
[2] Journal of Medicinal Chemistry, 1981, vol. 24, # 3, p. 341 - 346 |
|
|




![1,5-Dioxaspiro[5.5]undecan-9-ol, 3,3-dimethyl-](/CAS/20210305/GIF/69825-15-6.gif)
