| Identification | More | [Name]
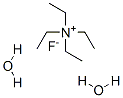
Tetraethylammonium fluoride dihydrate | [CAS]
665-46-3 | [Synonyms]
TETRAETHYLAMMONIUM FLUORIDE
Tetraethylammonium fluoride dihydrate
N,N,N-TRIETHYL-ETHANAMINIUFLUORIDE
Ethanaminium, N,N,N-triethyl-, fluoride
Tetraethylazanium fluoride
N,N,N-Triethylethanaminium·fluoride
Tetraethylaminium·fluoride | [EINECS(EC#)]
211-559-7 | [Molecular Formula]
C8H24FNO2 | [MDL Number]
MFCD00011826 | [Molecular Weight]
185.28 | [MOL File]
665-46-3.mol |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed .
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing . | [WGK Germany ]
3
| [F ]
3-9 | [HS Code ]
2921199990 |
| Hazard Information | Back Directory | [Uses]
Tetraethylammonium Fluoride Dihydrate is an analog of Tetraethylammonium Chloride (T292700). Tetraethylammonium Chloride can be used as a source of tetraethylammonium ions for various pharmaceutical studies and has the ability to block K+ channels in various tissues. Tetraethylammonium Chloride can also block the transmission of nervous impulses across autonomic ganglions. | [Synthesis]
(1) dissolve 10 g of tetraethylammonium iodide with 99% purity in 25 mL of distilled water, add 60 mL of hydrofluoric acid with a mass concentration of 35%, seal and heat at 50 °C with stirring; (2) ensure that the raw materials are fully reacted for at least 3 h, and then raise the temperature to 110 °C to evaporate the excess hydrogen fluoride gas and by-product hydrogen, and absorb the exhaust gas using calcium oxide, taking care to avoid tetraethylammonium fluoride ionic liquid dehydration; (3) diluting the undehydrated tetraethylammonium fluoride ionic liquid with distilled water, ensuring that the volume ratio of the tetraethylammonium fluoride ionic liquid to distilled water was less than 1:50. AgNO3 solution was added to the diluted solution, and if precipitation was observed to be generated, 20 mL of hydrofluoric acid was continued to be added, and steps (1) and (2) were repeated until no precipitate was generated in order to purify the tetraethylammonium fluoride ionic liquid; (4) The unpurified tetraethylammonium fluoride ionic liquid was dried under high vacuum at 120°C for at least 48 hours to remove water and residual hydrogen fluoride. The viscous liquid obtained needs to be diluted with distilled water to ensure that the volume ratio of viscous liquid to distilled water is less than 1:50, sealed and heated at 60°C, and the residual hydrogen fluoride concentration is detected using a hydrogen detector. If the reading is more than 0.1 ppm, then continue to dry in high vacuum at 120 ℃ until the concentration of hydrogen fluoride is less than 0.1 ppm When the concentration of hydrogen fluoride is detected to be less than 0.1 ppm, the resulting liquid is tetraethylammonium fluoride ionic liquid, and the weight of the final product is 5.67 g. The final product weighs 5.67 g. The final liquid is then dried in high vacuum at 120 ℃ until the concentration of hydrogen fluoride is less than 0.1 ppm. | [References]
[1] Patent: CN103992275, 2016, B. Location in patent: Paragraph 0264; 0265; 0266; 0267; 0268; 0269 |
|
|