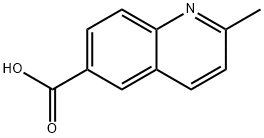
| Identification | More | [Name]
2-Methyl-6-quinolinecarboxylic acid | [CAS]
635-80-3 | [Synonyms]
2-METHYL-6-QUINOLINECARBOXYLIC ACID
2-METHYLQUINOLINE-6-CARBOXYLIC ACID
Quinaldine-6-carboxylic acid | [EINECS(EC#)]
251-785-3 | [Molecular Formula]
C11H9NO2 | [MDL Number]
MFCD00748504 | [Molecular Weight]
187.19 | [MOL File]
635-80-3.mol |
| Chemical Properties | Back Directory | [Melting point ]
250 °C | [Boiling point ]
347.6±27.0 °C(Predicted) | [density ]
1.285±0.06 g/cm3(Predicted) | [storage temp. ]
Sealed in dry,Room Temperature | [form ]
Solid | [pka]
3.13±0.30(Predicted) | [color ]
White to Light yellow to Light orange | [InChI]
InChI=1S/C11H9NO2/c1-7-2-3-8-6-9(11(13)14)4-5-10(8)12-7/h2-6H,1H3,(H,13,14) | [InChIKey]
IZONZQFTYGVOOO-UHFFFAOYSA-N | [SMILES]
N1C2C(=CC(C(O)=O)=CC=2)C=CC=1C | [CAS DataBase Reference]
635-80-3(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R22:Harmful if swallowed.
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S22:Do not breathe dust .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection . | [Hazard Note ]
Irritant | [HS Code ]
29334900 |
| Hazard Information | Back Directory | [Chemical Properties]
White solid | [Uses]
2-Methylquinoline-6-carboxylic Acid is a preparation of arylpyrazolopyridine derivative as monoacylglycerol lipase modulators. | [Synthesis]
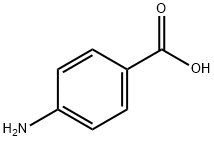
General procedure for the synthesis of 2-methyl-6-quinoline carboxylic acid from p-aminobenzoic acid and trans-2-butenal:
Intermediate 13: Synthesis of (2-methylquinolin-6-yl)methylamine:
Step 1: Preparation of 2-methylquinoline-6-carboxylic acid: 6N hydrochloric acid (73 ml) was added to 4-aminobenzoic acid (5 g, 36.45 mmol) and heated to reflux for 2 hours. Subsequently, crotonaldehyde (3.06 g, 43.75 mmol) was added slowly and dropwise over 45 min. After 12 hours of reaction, the reaction mixture was cooled to 0 °C and the pH was adjusted to 3-5 with aqueous ammonia solution. the aqueous phase was extracted with dichloromethane, the organic phases were combined and acidified with 2N hydrochloric acid, the precipitate was collected by filtration and dried under vacuum to afford 2-methyl-6-quinolinecarboxylic acid as a brown solid (3.0 g, 44% yield). | [References]
[1] Patent: WO2013/144737, 2013, A2. Location in patent: Paragraph 252
[2] Patent: US2015/57309, 2015, A1. Location in patent: Paragraph 0524
[3] Journal of Medicinal Chemistry, 2002, vol. 45, # 21, p. 4647 - 4654
[4] Chemistry - A European Journal, 2018, vol. 24, # 61, p. 16368 - 16378
[5] Bioorganic and Medicinal Chemistry Letters, 2007, vol. 17, # 22, p. 6138 - 6141 |
|
|