| Identification | More | [Name]
2-Methyl-5-nitrobenzenesulfonamide | [CAS]
6269-91-6 | [Synonyms]
2-METHYL-5-NITROBENZENESULFONAMIDE
2-METHYL-5-NITROBENZENESULPHONAMIDE
BUTTPARK 52\04-61
IFLAB-BB F1084-0158
4-Nitro-2-toluenesulfonamide
BENZENESULFONAMIDE,2-METHYL-5-NITRO-
2-methyl-5-nitrobenzene-1-sulfonamide
2-Methyl-5-nitro-1-benzenesulfonamide | [EINECS(EC#)]
220-358-3 | [Molecular Formula]
C7H8N2O4S | [MDL Number]
MFCD00115503 | [Molecular Weight]
216.21 | [MOL File]
6269-91-6.mol |
| Chemical Properties | Back Directory | [Melting point ]
197-199 | [Boiling point ]
431.4±55.0 °C(Predicted) | [density ]
1.475±0.06 g/cm3(Predicted) | [storage temp. ]
2-8°C | [solubility ]
Chloroform (Sparingly), Methanol (Slightly) | [form ]
Solid | [pka]
9.56±0.60(Predicted) | [color ]
Yellow to Dark Yellow | [CAS DataBase Reference]
6269-91-6(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Uses]
2-Methyl-5-nitrobenzenesulfonamide is a chemical reagent in the synthesis of good inhibitors of cancer-related carbonic anhydrase. Also used in the synthesis of novel deacetylase inhibitors used in anti-tumor therapy. | [Synthesis]
Step B: 2-methyl-5-nitrobenzenesulfonyl chloride (10.0 g, 43.7 mmol) was dissolved in ether (300 mL) under nitrogen protection and cooled to 0 °C. Ammonia (40 mL) was added slowly and the reaction mixture was stirred at 0 °C for about 42 hours. After the reaction was complete, the mixture was filtered and diluted with dichloromethane (600 mL). The organic layer was separated and dried with anhydrous magnesium sulfate. The organic layer was concentrated under reduced pressure to give 2-methyl-5-nitrobenzenesulfonamide (9.2 g, 100% yield).1H NMR (DMSO-d6) δ: 8.60 (d, J = 2.4Hz, 1H), 8.34 (dd, J = 8.0,2.4Hz, 1H), 7.77 (br s, 2H), 7.70 (d, J = 8.4Hz, 1H), 7.70 (d, J = 8.4Hz, 1H). 2.71 (s, 3H). | [structure and hydrogen bonding]
In the 2-Methyl-5-nitrobenzenesulfonamide, C7H8N2O4S, the nitro group is twisted by 9.61 (2)° relative to the benzene ring. In the crystal, mol-ecules are linked by N—H…O and N—H…(O, O) hydrogen bonds between the amino and sulfonyl groups, forming layers parallel to (001)[1].
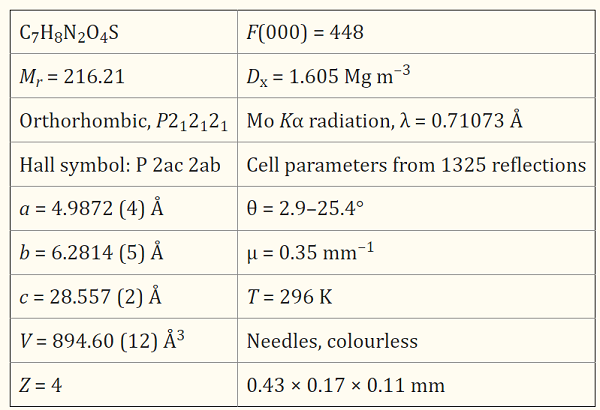 | [References]
[1] Muhammad Zia-Ur-Rehman. “2-Methyl-5-nitro-benzene-sulfonamide.” Acta crystallographica. Section E, Structure reports online 66 Pt 1 (2009): o136.
|
|
|