| Identification | Back Directory | [Name]
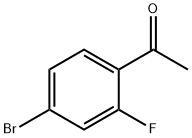
4-BROMO-2-FLUOROACETOPHENONE | [CAS]
625446-22-2 | [Synonyms]
4-BROMO-2-FLUOROACETOPHENONE
2-Fluoro-4-bromoacetophenone
4'-BROMO-2'-FLUOROACETOPHENONE
1-Acetyl-4-bromo-2-fluorobenzene
4'-Bromo-2'-fluoroacetophenone98%
4'-Bromo-2'-fluoroacetophenone 98%
4'-Bromo-2'-fluoroacetophenone,96%
1-(4-Bromo-2-fluorophenyl)ethan-1-one
1-(4-broMophenyl)-2-fluoroethan-1-one
Ethanone, 1-(4-broMo-2-fluorophenyl)-
4-Bromo-2-fluoroacetophenone/2-Fluoro-4-bromoacetophenone | [EINECS(EC#)]
205-516-1 | [Molecular Formula]
C8H6BrFO | [MDL Number]
MFCD03411551 | [MOL File]
625446-22-2.mol | [Molecular Weight]
217.04 |
| Chemical Properties | Back Directory | [Melting point ]
23 °C | [Boiling point ]
256.3±25.0 °C(Predicted) | [density ]
1.535±0.06 g/cm3(Predicted) | [storage temp. ]
Sealed in dry,Room Temperature | [solubility ]
Acetonitrile (Slightly), Chloroform (Slightly) | [form ]
Solid | [color ]
White to Off-White | [InChI]
InChI=1S/C8H6BrFO/c1-5(11)7-3-2-6(9)4-8(7)10/h2-4H,1H3 | [InChIKey]
ASKFCSCYGAFWAB-UHFFFAOYSA-N | [SMILES]
C(=O)(C1=CC=C(Br)C=C1F)C |
| Hazard Information | Back Directory | [Synthesis]
To a solution of 4-bromo-2-fluoro-N-methoxy-N-methylbenzamide (13 g, 46 mmol) in THF (4.0 mL) was added dropwise methylmagnesium bromide (3M ethyl ether solution, 30 mL, 91 mmol) at 0°C, and the mixture was stirred at room temperature for 3 hr. For the reaction mixture, saturated aqueous ammonium chloride solution, and the mixture was extracted with ethyl acetate. The obtained extract was washed with water and saturated brine, and dried over anhydrous magnesium sulfate. The solvent was evaporated under reduced pressure to give 4-Bromo-2-fluoroacetophenone as a pale yellow oil. | [References]
[1] Patent: US2008/45556, 2008, A1. Location in patent: Page/Page column 32
[2] Patent: US2011/118257, 2011, A1. Location in patent: Page/Page column 97-98
[3] Patent: US2011/281865, 2011, A1. Location in patent: Page/Page column 85-86
[4] Patent: WO2011/145035, 2011, A1. Location in patent: Page/Page column 114-115
[5] Journal of Medicinal Chemistry, 2008, vol. 51, # 2, p. 282 - 297 |
|
|