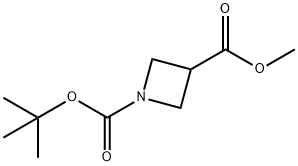
| Identification | Back Directory | [Name]
AZETIDINE-1,3-DICARBOXYLIC ACID 1-TERT-BUTYL ESTER 3-METHYL ESTER | [CAS]
610791-05-4 | [Synonyms]
N-Boc-3-a
3-dicarboxylate
1-Boc-azetidine-3-carboxyL
Methyl 1-Boc-azetidine-3-...
tert-butyl Methyl azetidine-1
Methyl 1-Boc-azetidine-3-carboxylate
Methyl 1-Boc-azetidine-3-carboxylate 95%
TERT-BUTYL METHYL AZETIDINE-1,3-DICARBOXYLATE
1-BOC-AZETIDINE-3-CARBOXYLIC ACID METHYL ESTER
N-Boc-3-azetidine carboxylic acid methyl ester
Methyl azetidine-3-carboxylate, N-BOC protected
1-tert-butyl 3-methyl azetidine-1,3-dicarboxylate
1-O-tert-butyl 3-O-methyl azetidine-1,3-dicarboxylate
AZETIDINE-1,3-DICARBOXYLIC ACID 1-TERT-BUTYL ESTER 3-METHYL ESTER
1-(tert-Butoxycarbonyl)-3-(methoxycarbonyl)azetidine, 1-tert-Butyl 3-methyl azetidine-1,3-dicarboxylate | [Molecular Formula]
C10H17NO4 | [MDL Number]
MFCD06656775 | [MOL File]
610791-05-4.mol | [Molecular Weight]
215.25 |
| Chemical Properties | Back Directory | [Boiling point ]
269.0±33.0 °C(Predicted) | [density ]
1.072 g/mL at 25 °C | [refractive index ]
n20/D1.452 | [Fp ]
>110℃ | [storage temp. ]
Keep in dark place,Sealed in dry,Room Temperature | [form ]
Liquid | [pka]
-2.97±0.40(Predicted) | [color ]
Colorless to light yellow | [InChI]
InChI=1S/C10H17NO4/c1-10(2,3)15-9(13)11-5-7(6-11)8(12)14-4/h7H,5-6H2,1-4H3 | [InChIKey]
SECFRXGVLMVUPD-UHFFFAOYSA-N | [SMILES]
N1(C(OC(C)(C)C)=O)CC(C(OC)=O)C1 |
| Hazard Information | Back Directory | [Uses]
Building block used as a precursor to the synthesis of azaspiro[3.4]octanes developed in the Carreira group. This substrate can be readily functionalized via enolization at the 3-position in the presence of LDA. | [Synthesis]
General procedure for the synthesis of methyl 1-(tert-butoxycarbonyl)azetidine-3-carboxylate from methanol and 1-Boc-azetidine-3-carboxylic acid: 1-(tert-butoxycarbonyl)azetidine-3-carboxylic acid (AXL016917, 1000 mg, 4.97 mmol) was dissolved in a mixture of solvents with methanol (5 mL) and toluene (20 mL), and subsequently the solution was cooled to 0°C. Trimethylsilyl diazomethane (7.45 mmol) was slowly added dropwise over a period of 15 min, during which slight bubble production was observed. The color of the solution gradually changed from clear to light yellow. The reaction mixture was continued to be stirred at 0 °C for 10 min, followed by slow warming to room temperature over a period of 30 min. Upon completion of the reaction, toluene was removed by concentration and distillation under reduced pressure to afford 1.055 g of methyl 1-(tert-butoxycarbonyl)azetidine-3-carboxylate (99% crude yield), which could be used in the next step of the reaction without further purification. | [IC 50]
Non-cleavable Linker | [References]
[1] Patent: WO2006/21881, 2006, A2. Location in patent: Page/Page column 61
[2] Patent: WO2013/83991, 2013, A1. Location in patent: Page/Page column 69
[3] Patent: WO2012/142237, 2012, A1. Location in patent: Page/Page column 98-99 |
|
|